Question
Question: The correct stability order of the following resonance structure is: (I)  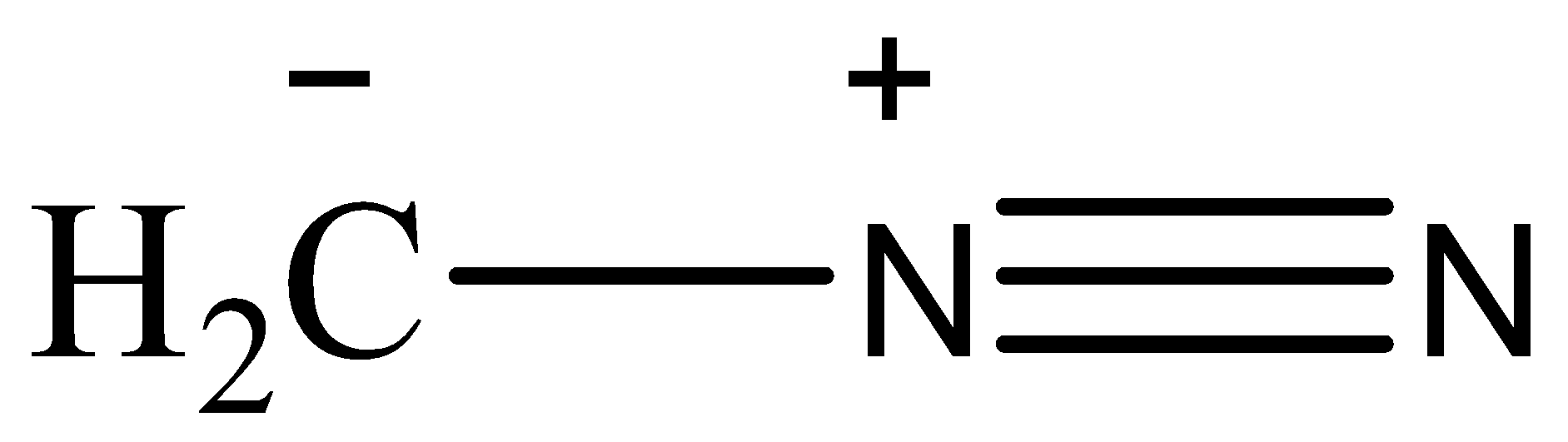
(II) 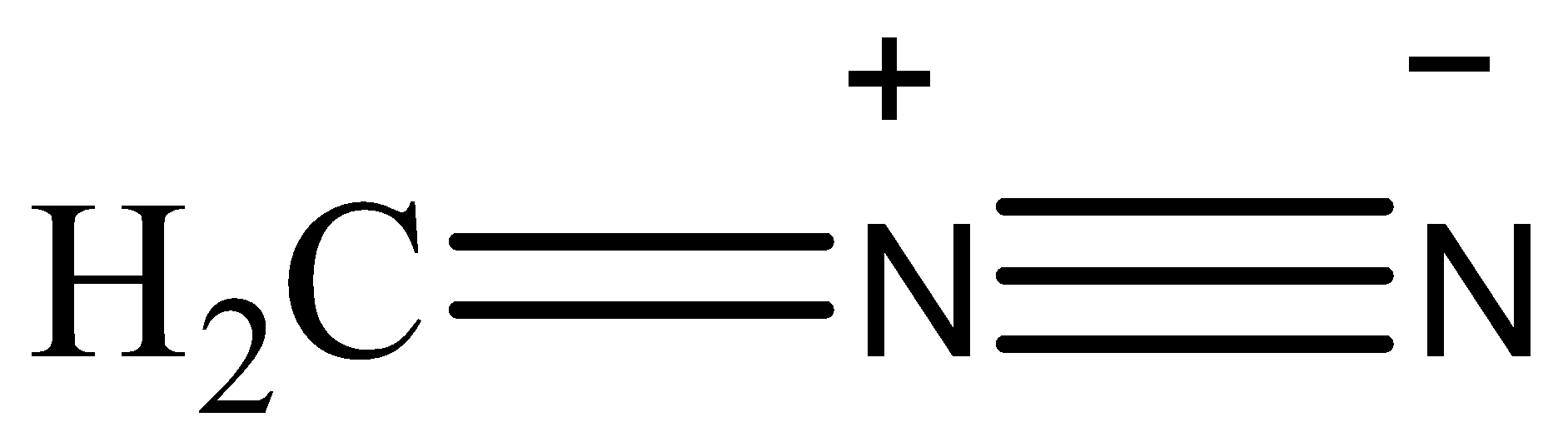
(III) 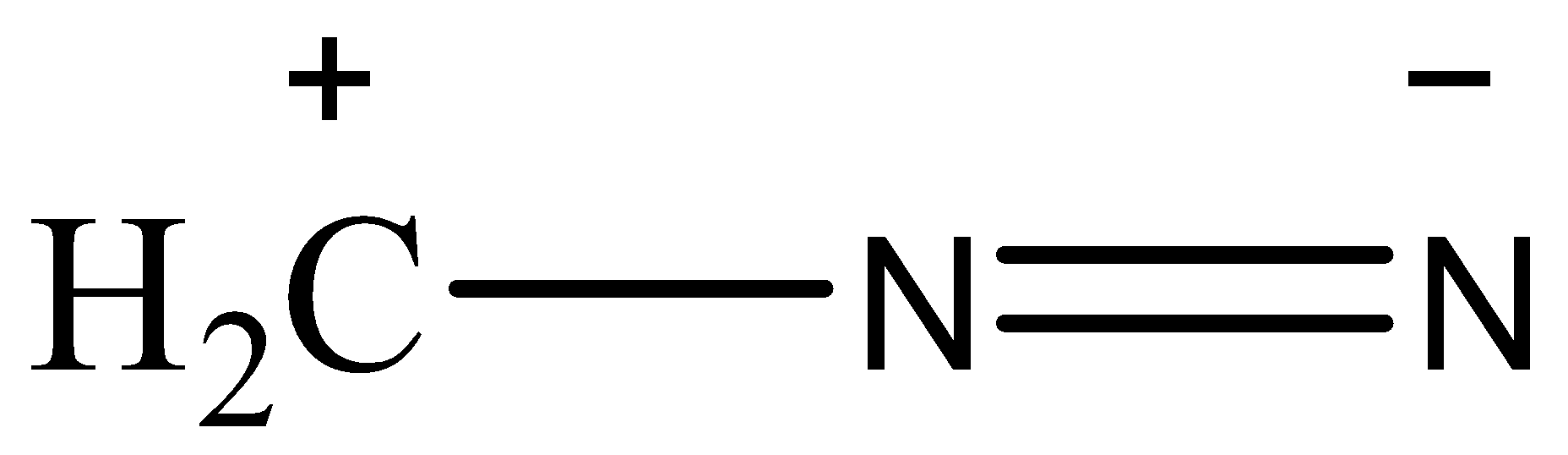
(IV) 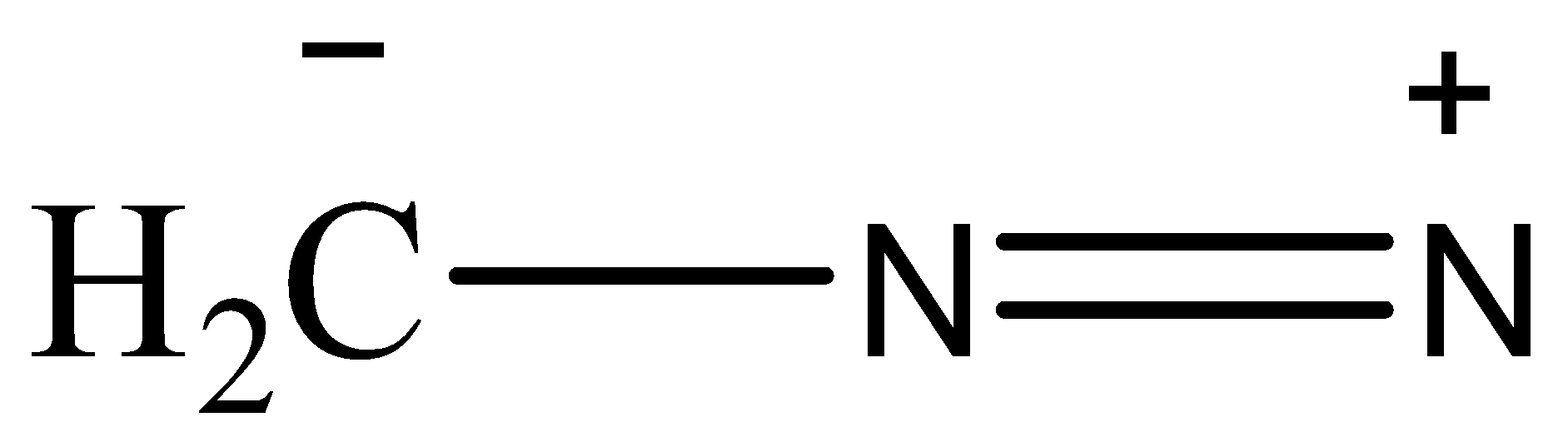
(A) (I)>(II)>(IV)>(III)
(B) (I)>(III)>(II)>(IV)
(C) (II)>(I)>(III)>(IV)
(D) (III)>(I)>(IV)>(II)
Solution
Hint : Resonance effect arises due to the polarity that is produced in a molecule due to interaction between a lone pair and a π bond electron. It can also arise when there is π−π conjugated system i.e. interaction of two pi bonds between two adjacent atoms.
Complete step by step solution:
In chemistry, Resonance is the phenomenon in which a molecule can move its π electrons in the system. These electrons delocalized themselves in the compound forming a conjugated system of π electrons. This movement of the electrons cannot be exhibited with the help of one lewis structure, hence more than one structure is drawn to explain the delocalization. These structures are called resonating structures.
Now, the stability of the resonating structures depends upon many factors. Now, we will discuss these factors and then arrange the given resonating structures according to their stability. The resonance structures that have a complete octet are more stable than the ones whose octet are incomplete. The placement of charge also affects the stability, if a negative charge is placed on an electronegative atom then the structure will be more stable as the atom will balance the charge. Charges should be separated and the point charges should be minimum. A neutral species is more stable than a charged species. The structure that has the most number of covalent bonds will be the most stable.
Based on these observations, the correct order of stability will be (II)>(I)>(III)>(IV)
Therefore, option (C) is correct.
Note:
Resonance in chemistry is also used to explain the bonding in some particular molecules or ions by merging many structures, which are called canonical structures or resonance structures with the help of valence bond theory into a hybrid resonance which is called hybrid structures.
