Question
Question: The correct sequence of steps involved in the mechanism of Cannizzaro reaction is: A. nucleophilic...
The correct sequence of steps involved in the mechanism of Cannizzaro reaction is:
A. nucleophilic attack, transfer of H− and transfer of H+
B. transfer of H−, transfer of H+ and nucleophilic attack
C. transfer of H+ nucleophilic attack and transfer of H−
D. electrophilic attack by OH− transfer ofH− and transfer of H−
Solution
Cannizzaro reaction is a reaction in which disproportionation of two molecules of aldehyde takes place to give a primary alcohol and a carboxylic acid, it involves the nucleophilic substitution on aldehydes.
Complete step by step answer:
- We can see from the following mechanism that how Cannizzaro reaction takes place:
- We can see that in step 1, we have, one HCHO and one OH−, which will react together and this will be the fast step of the reaction, we can say that there is nucleophilic attack of OH− to the carbonyl carbon and we get:
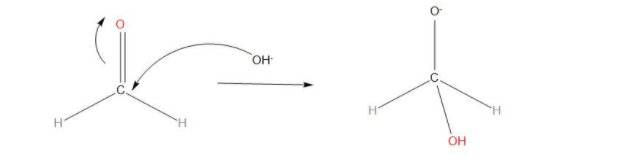
- In step 2, we can see that the transfer of hydride ions from anion to the second molecule of aldehyde takes place. And finally the rapid transfer of protons takes place.
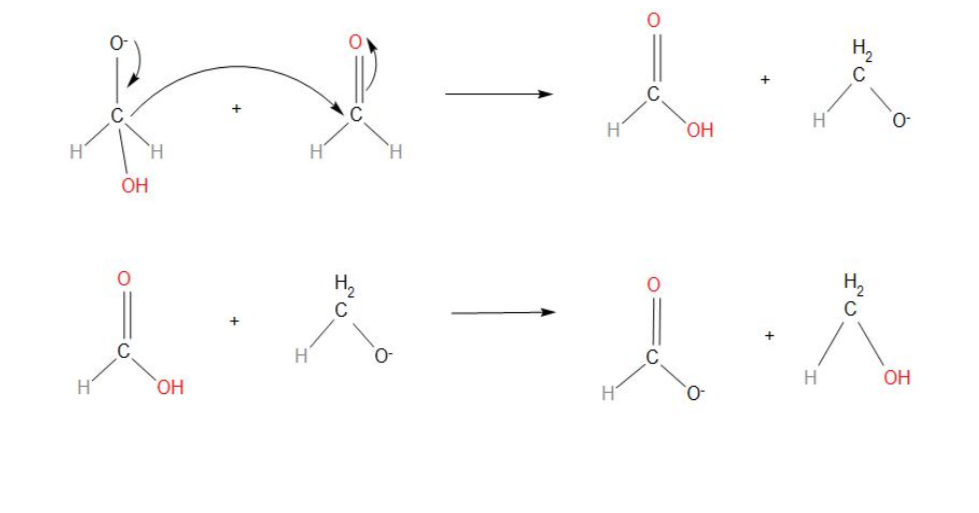
- Hence, we can conclude that the correct sequence of steps involved in the mechanism of Cannizzaro reaction is: nucleophilic attack, transfer of H− and transfer of H+
So, the correct answer is “Option C”.
Note: - This reaction is always given by aldehydes that doesn’t have an alpha hydrogen atom (it is present on the alpha carbon). For example, acetaldehyde doesn’t undergo a cannizzaro reaction because it has alpha hydrogen.
