Question
Question: The correct product is 
a.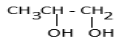
b.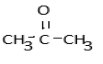
c.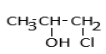
d.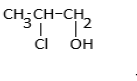
Solution
Alkenes are the electron rich species. They have double bond with π electrons. In many reaction hence they act as strong nucleophilic centres and attack the electrophiles. After that formation of stable intermediate (carbocation, radical, etc.) occurs which leads to the product.
Complete step-by-step answer: Alkenes are the electron rich species. They have one double bond because of which in many reaction they acts as strong nucleophilic centres and attack the electrophiles. After that formation of stable intermediate occurs which lead us to the product.
A very famous reaction where alkene acts as nucleophile are the Markonikov;s reaction where in the reaction of unsymmetrical alkene with hydrogen bromide, the carbon with more hydrogen gets hydrogen and the other one gets the bromine atom.
CH3−CH=CH2+HBr→CH3−CH(Br)−CH3
In the process when chlorine is poured in water, the chlorine undergoes hydrolysis and the formation of hypochlorous acid takes place. The hypochlorous acid thus formed is very reactive in nature and so it readily reacts with alkene.
Another method that can act here will be, as we know the alkene has one double bond so the nucleophilicity of alkene is high hence it attacks the Clδ+−Clδ− bond. The bond then breaks and Cl− leaves. The other chlorine forms an intermediate with positive charge in it. Now the lone pair on oxygen present in water attacks the more electrophilic carbon and the intermediate breaks into the product.
The mechanism can be drawn as the following-
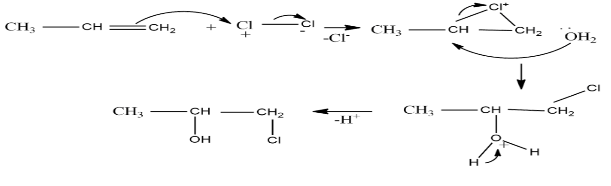
Therefore, the correct option is (c).
Note: Alkanes on reaction with chlorine in presence of heat undergoes free radical reactions. Their chlorine free radical forms and they attack on carbon hydrogen form forming carbon chlorine bonds. In this method we can get the mono-substituted product or the poly-substituted product as we cannot control radical reactions.
