Question
Question: The correct orientation of dipoles in pyrrole and pyridine is: (a) 
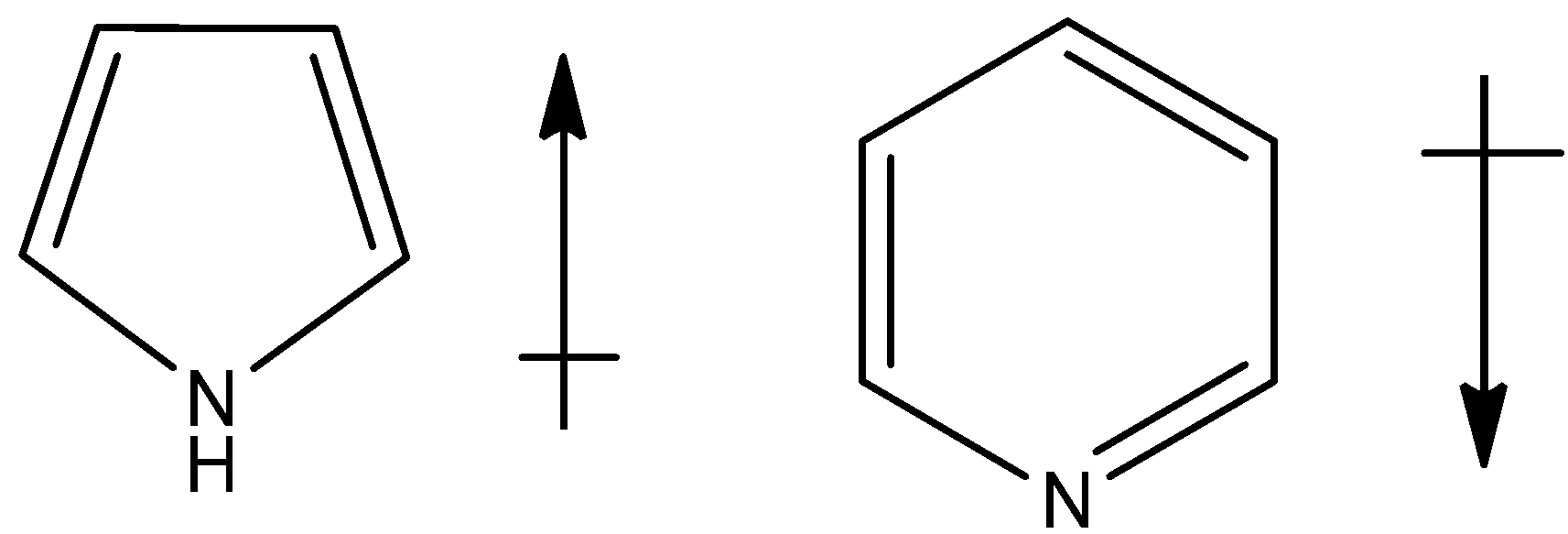
(b)
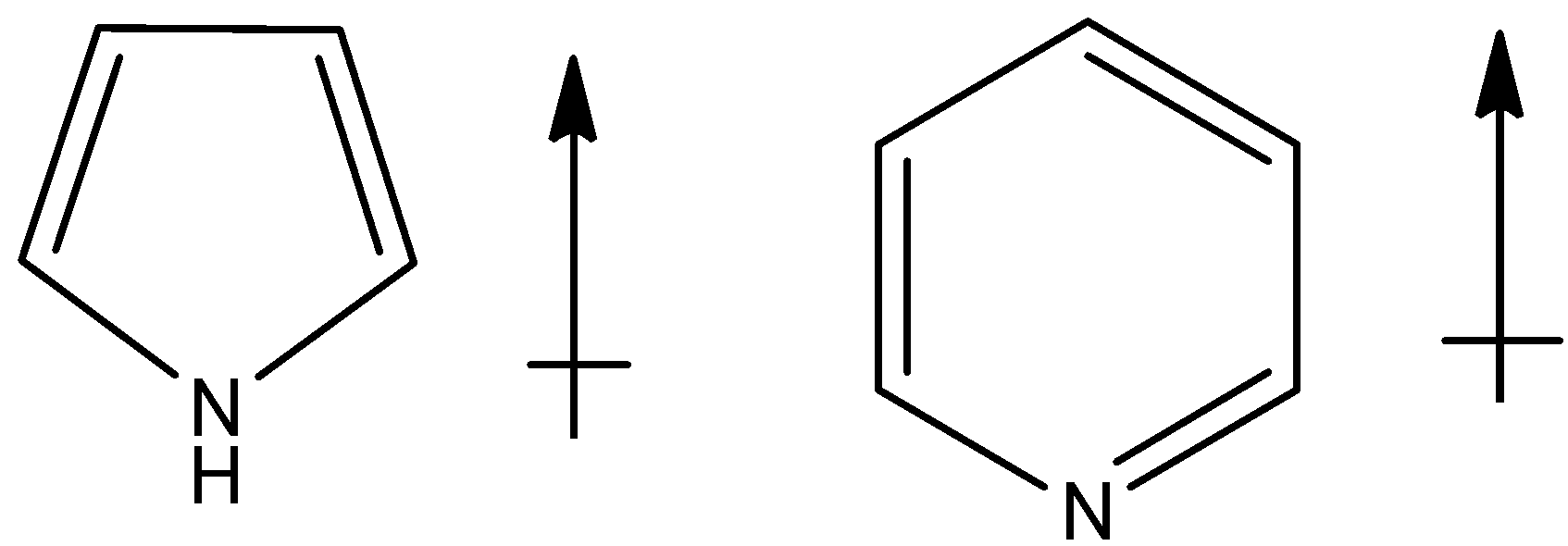
(c)
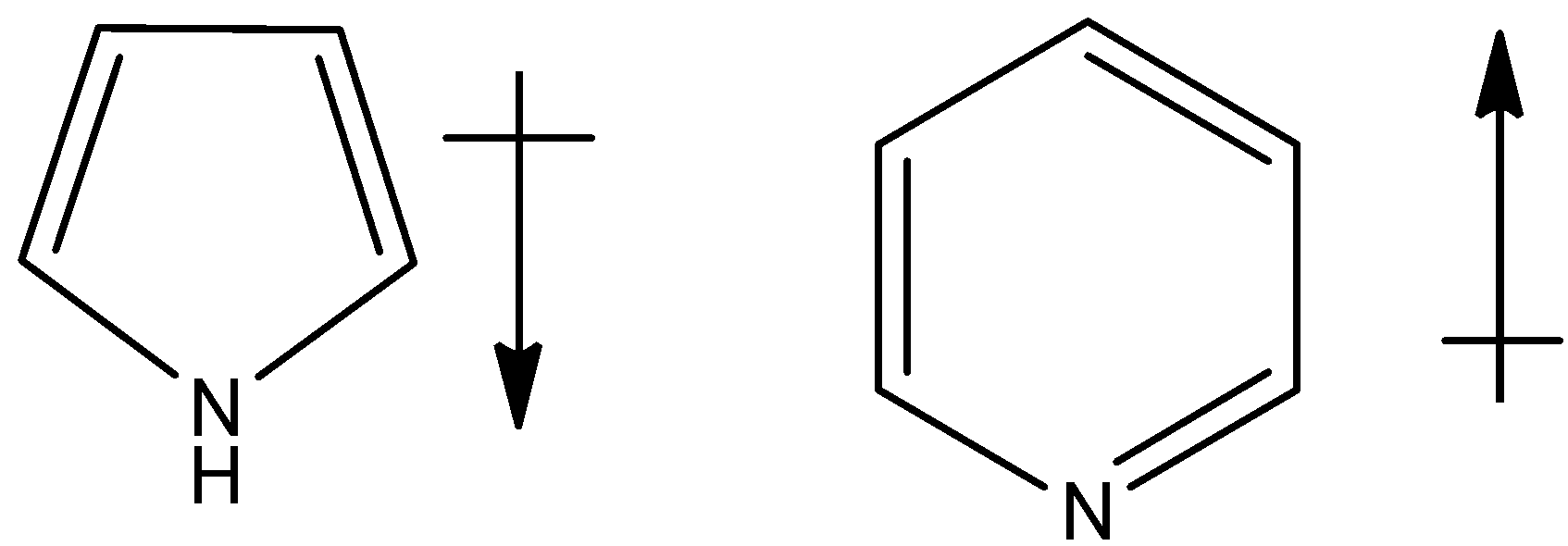
(d)
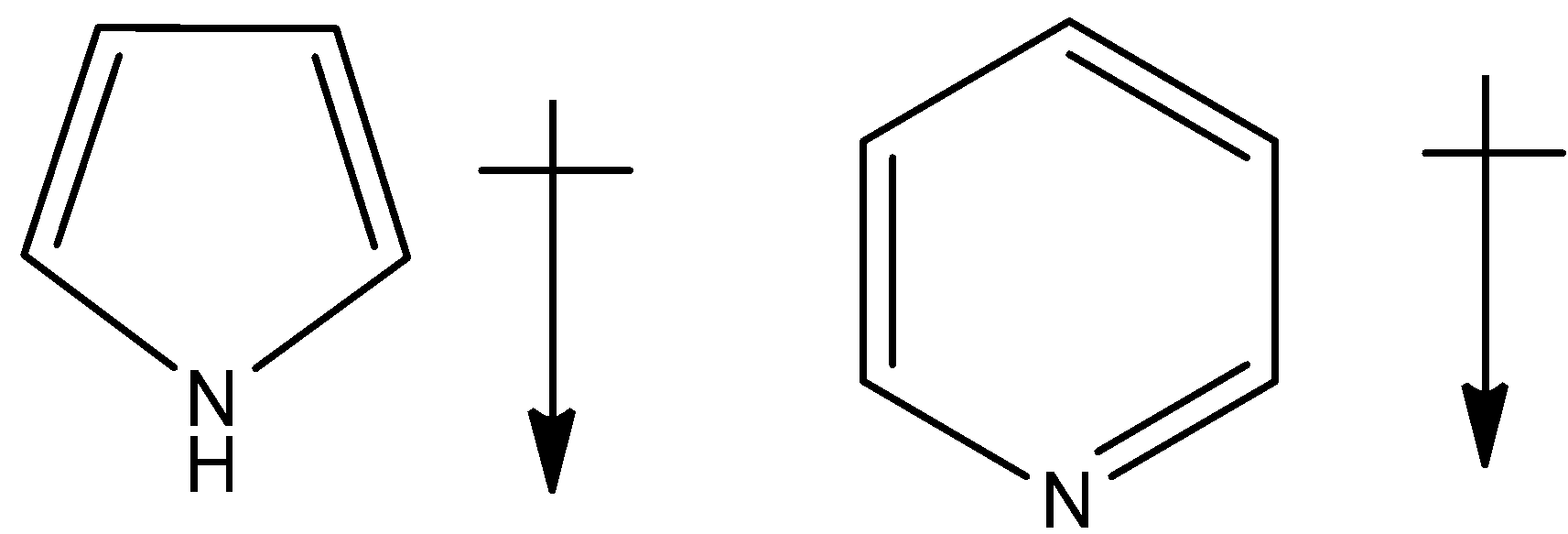
Solution
The answer to this question lies in the basic concept of chemistry which tells that dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together.
Complete step by step answer:
In the classes of chemistry, we have studied the basic concepts of organic chemistry that tells us about the dipole moment of a molecule and its measurement and also several related parameters.
Now, we will see what does dipole mean and how can it be represented.
- In chemistry, the definition of the dipole moment is given as the measure of the polarity of the chemical bond within a molecule and this occurs whenever there is a separation of positive and negative charges.
- Dipole moment can be measured using the formula as well as the arrow mark direction where the dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together.
- In the above given molecule, let us see the direction of the dipole moment in both the molecules one by one.
- In pyrrole molecule, the electronegativity of the carbon is less when compared to the nitrogen and therefore the dipole points towards the nitrogen and the net dipole points downwards within the molecule as shown below,
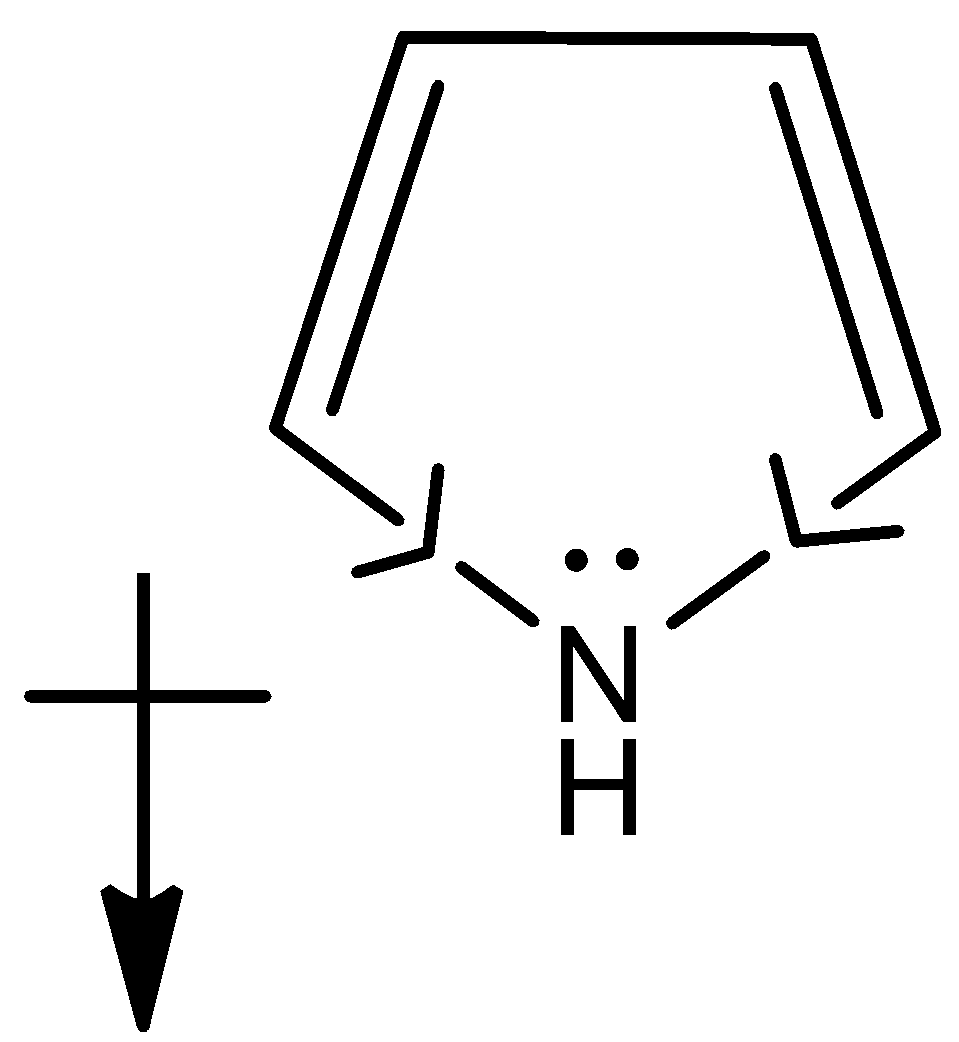
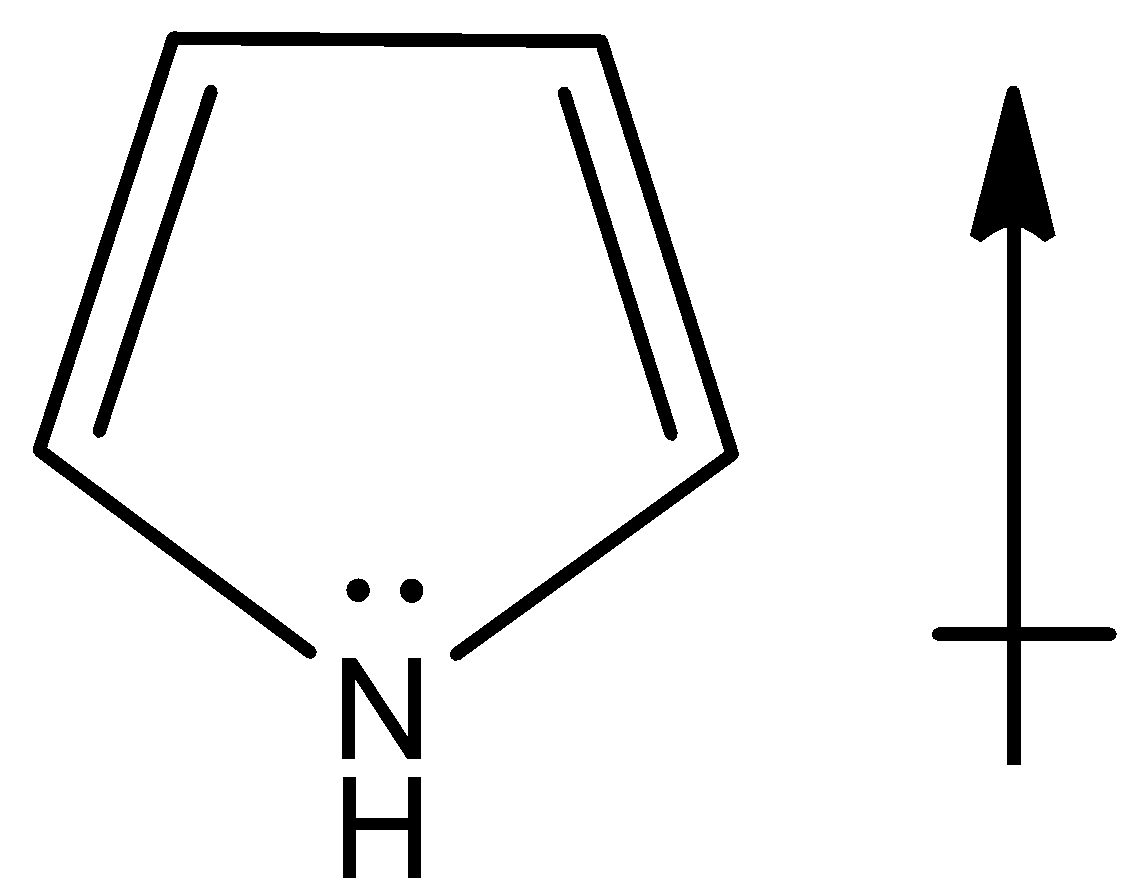
Now, since the lone pair of electrons present on the nitrogen atom points upwards as it is present on sp3 hybridized atom and also the hydrogen bonded to it will be having the upward direction of dipole. Thus net dipole will be in the upward direction,
In case of pyridine, even here the electronegativity of the carbon is less that nitrogen and the net dipole will be in downward direction,
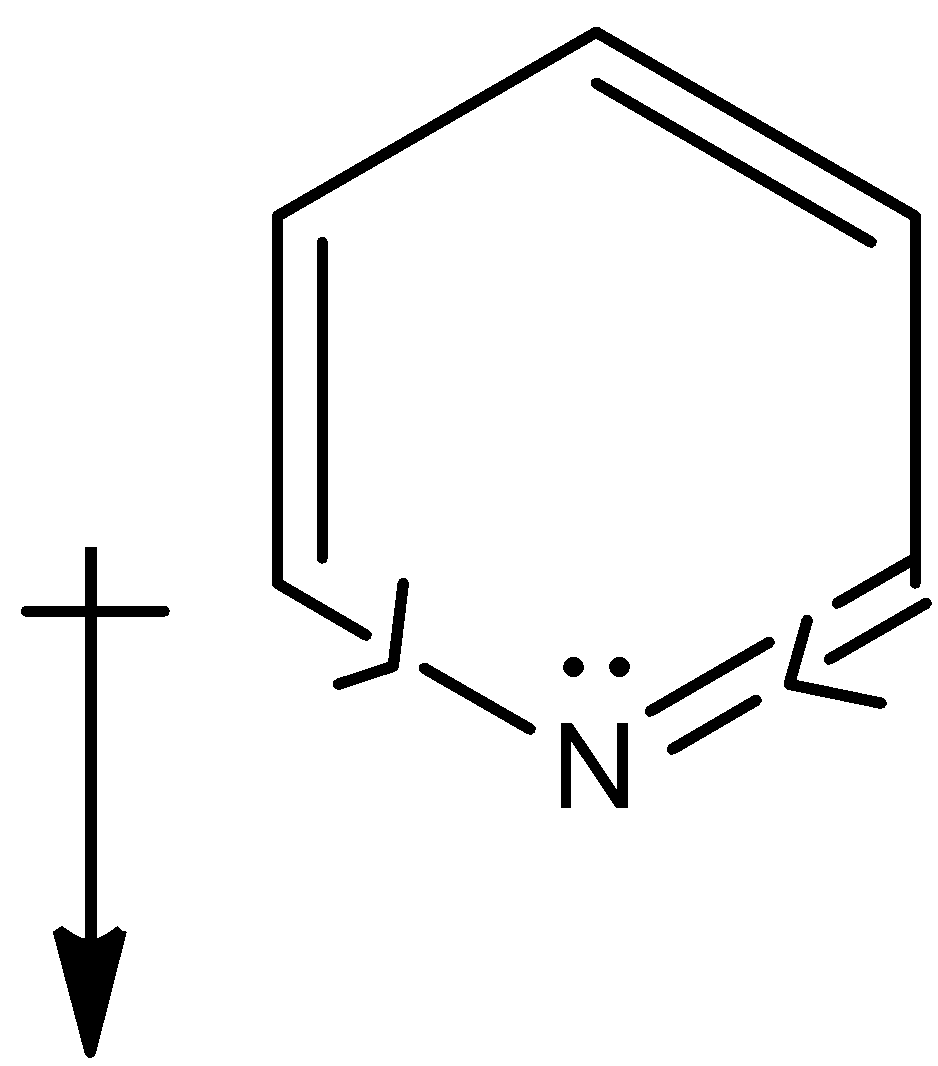
But, the lone pair of electrons on the nitrogen does not participate in the resonance as in case of pyrrole because the lone pair is present in the sp2 hybridised orbital which protrudes out of ring perpendicular to the pi bond system.
The correct option is option “A” .
Note: Note that direction of the dipole moment of lone pairs in pyrrole points upwards because it is involved in the resonance with the ring and the compound is aromatic and thus the net dipole moment will be in upward direction.
