Question
Question: The correct order of the \(O-O\) bond length in \({{O}_{2}}\), \({{H}_{2}}{{O}_{2}}\) and \({{O}_{3}...
The correct order of the O−O bond length in O2, H2O2 and O3 is
(1) O2>H2O2>O3
(2) H2O2>O3>O2
(3) O2>O3>H2O2
(4) O3>H2O2>O2
Solution
bond order is nothing but total number of bonds present in between the two atoms. The required answer includes concepts based on the molecular orbital theory where bond order is inversely proportional to bond length.
Complete step by step solution:
We have studied in our inorganic chemistry part that includes the chapter of finding bond order and also the structure of atoms based on several theories.
- One among those theories includes the molecular orbital theory which is well known as MOT.
- Molecular orbital theory is based on the method to depict the diagrams on the basis of their electronic structure using quantum mechanics.
- Molecular orbital theory makes use of the linear combination of atomic orbital
(LCAO).
- The diagram is based on three types of bonding. They are, bonding, antibonding and the non-bonding atomic orbital.
Now, according to the question we must find the order of bond length with the data given in terms of bond order. For this, let us draw the structures of the molecules given which is as shown below,
Structure of O2 is as follows,

Bond order = 2
Structure ofH2O2 is given below,
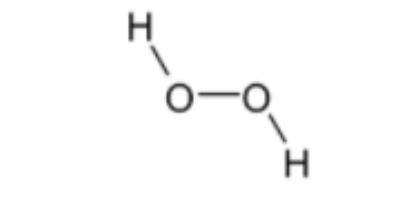
Bond order = 1
Structure of O3 is,
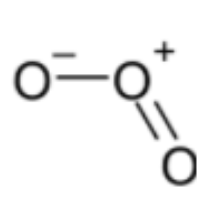
Bond order = 1.5
Therefore, increasing order of bond order is H2O2<O3<O2
Since, the bond angle is inversely proportional to bond order we can write the decreasing order of bond length as,
H2O2>O3>O2
Thus, the correct answer is option (B).
Note: The principle of valence bond theory (VBT) and molecular orbital theory (MOT) are the same and not to be confused. But MOT prevails as it was successful in explaining the paramagnetic character of oxygen but not VBT.
