Question
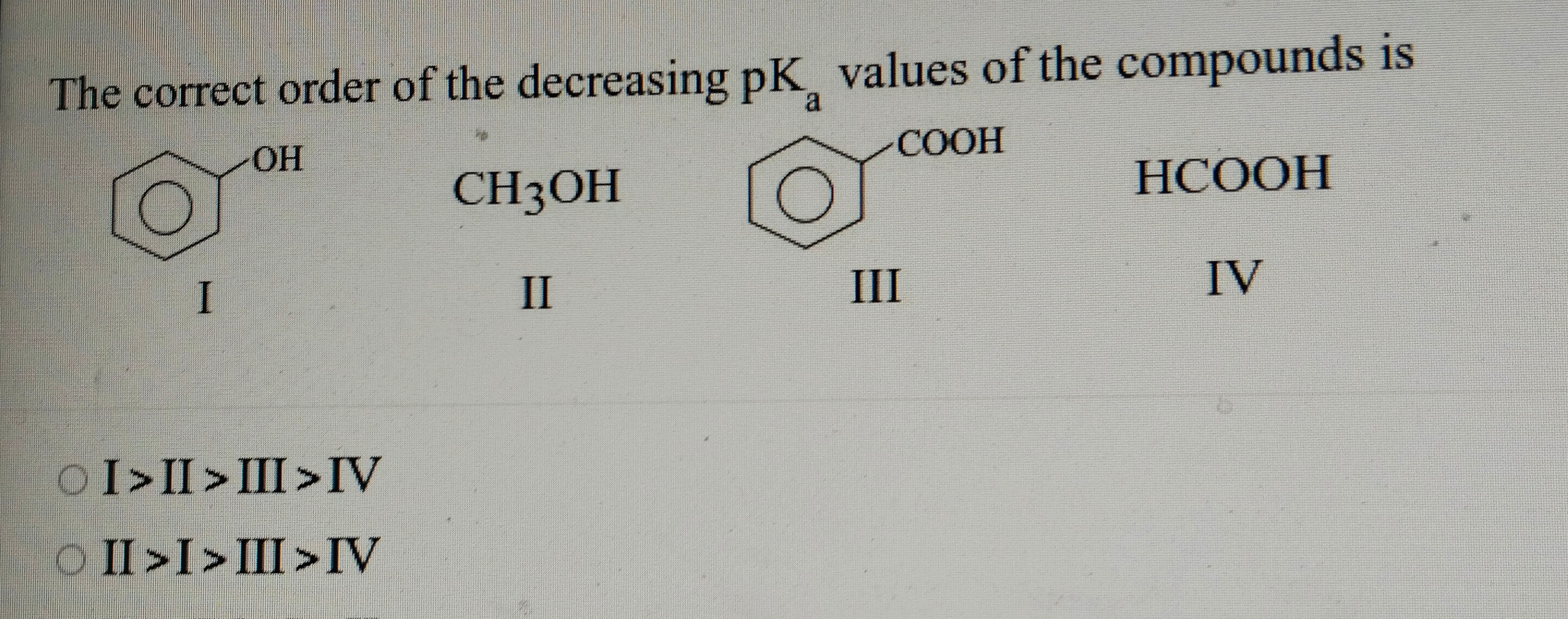
Question: The correct order of the decreasing pK\textsubscript{a} values of the compounds is ...
The correct order of the decreasing pK\textsubscript{a} values of the compounds is
A
I>II>III>IV
B
II>I>III>IV
Answer
II > I > III > IV
Explanation
Solution
The pKa values are approximately:
-
Methanol (II): pKa ≈ 16
-
Phenol (I): pKa ≈ 10
-
Benzoic acid (III): pKa ≈ 4.2
-
Formic acid (IV): pKa ≈ 3.75
Since a lower pKa indicates a stronger acid, the order from the highest pKa (weak acid) to the lowest pKa (strong acid) is: Methanol (II) > Phenol (I) > Benzoic acid (III) > Formic acid (IV)
