Question
Question: The correct order of ‘\({\text{S}} - {\text{O}}\)’ bond length is- (A) \({\text{SO}}_3^{2 - } > {\...
The correct order of ‘S−O’ bond length is-
(A) SO32−>SO42−>SO3>SO2
(B) SO32−>SO42−>SO2>SO3
(C) SO42−>SO32−>SO2>SO3
(D) SO42−>SO32−>SO3>SO2
Solution
The average distance between the nuclei of the two bonded atoms is known as bond length.The number of covalent bonds in any molecule is known as its bond order. The bond length is inversely proportional to the bond order.
Formulae used:
Bond order=Total number of canonical formTotal number of bonds between two atoms
Bond length∝Bond order1
Complete step by step solution:
We are given four species SO32−, SO42−, SO3 and SO2.
Determine the bond order of SO32− as follows:
The structure of SO32− is as follows:
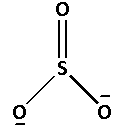
The number of bonds between the atoms in SO32− is 4. The number of canonical forms of SO32− is 3. The bond order of SO32− is,
Bond order of SO32−=34=1.33
Thus, the bond order of SO32− is 1.33.
Determine the bond order of SO42− as follows:
The structure of SO42− is as follows:
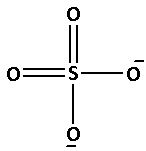
The number of bonds between the atoms in SO42− is 6. The number of canonical forms of SO42− is 4. The bond order of SO42− is,
Bond order of SO42−=46=1.5
Thus, the bond order of SO42− is 1.5.
Determine the bond order of SO3 as follows:
The structure of SO3 is as follows:
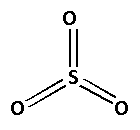
The number of bonds between the atoms in SO3 is 6. The number of canonical forms of SO3 is 3. The bond order of SO3 is,
Bond order of SO3=36=2
Thus, the bond order of SO3 is 2.
Determine the bond order of SO2 as follows:
The structure of SO2 is as follows:
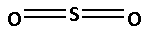
The number of bonds between the atoms in SO2 is 6. The number of canonical forms of SO2 is 3. The bond order of SO2 is,
Bond order of SO2=24=2
Thus, the bond order of SO2 is 2.
Thus, the decreasing order of bond order is,
SO3=SO2>SO42−>SO32−
The bond orders of SO3 and SO2 are same. But the bond pair-bond pair repulsion is higher in SO3 and thus, the S−O bond length in SO3 is smaller than that in SO2.
The bond length is inversely proportional to the bond order. Thus, the decreasing order of bond length is,
SO32−>SO42−>SO2>SO3
Thus, the correct option is (B) SO32−>SO42−>SO2>SO3.
Note: The bond orders of SO3 and SO2 are same. But the bond pair-bond pair repulsion is higher in SO3 and thus, the S−O bond length in SO3 is smaller than that in SO2.
