Question
Question: The correct order of strength of the carboxylic acids is 
(A) III>II>I
(B) II>I>III
(C) I>II>III
(D) II>III>I
Solution
Carboxylic acid is acidic in nature. It is a weak acid. Strength of acid is the tendency of a substance to release H+.If dissociation of acid is completed in an aqueous solution, then it is a strong acid. But carboxylic acid does not dissociate completely in an aqueous solution.
Complete answer:
Carboxylic Acid is a weak acid. But groups attached to –COOH differ in the strength of acid R-COOH.
If the number of C-atoms in the alkyl group increases, the acidity of carboxylic acid decreases.
HCOOH<CH3COOH<CH3CH2COOH<CH3(CH2)2COOH
If an electron withdrawing substance is present on a C-atom attached to−COOH, acidity increases.
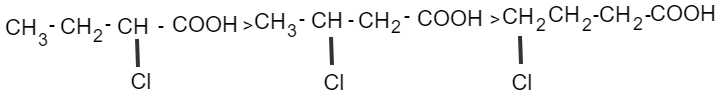
More electronegative atom increases the acidity of carboxylic acid.
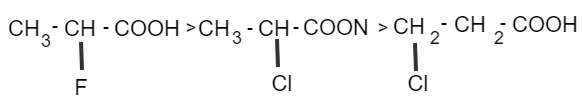
Acidity increases as the electronegativity of C-atom, directly attached to –COOH group increases or hybridization change from sp3→sp2→sp
HC≡C−COOH>CH2=CHCOOH>CH3COOH
In the given example –COOH is attached to sp3 C-atom and sp3 hybridized carbon attached to electronegative atoms (oxygen).
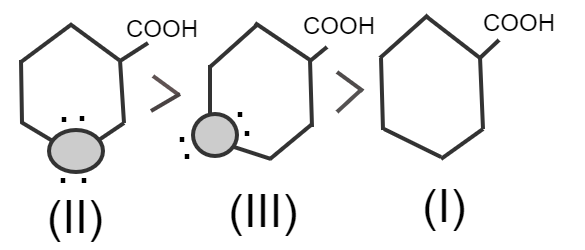
Electronegative atoms of oxygen increase polarity on the –COOH group, so it releases H+easily.
In structure (III) oxygen is away from –COOH so it produces less polarity on the –COOH group, which makes carboxylic acid weaker.
In structure (I) there is no electronegative atom so this is the weakest acid.
Therefore, from the above explanation, the correct option is (D) II>III>I.
Note: Carboxylic acid is a weak acid but the presence of substituents affects acidity. Electron withdrawing groups increase acidity and electron releasing groups decrease the acidity of carboxylic acid.
