Question
Question: The correct order of stability of the superoxides is: A. \[K{O_2} > Rb{O_2} > Cs{O_2}\] B. \[K{...
The correct order of stability of the superoxides is:
A. KO2>RbO2>CsO2
B. KO2>CsO2>RbO2
C. CsO2>RbO2>KO2
D. RbO2>CsO2>KO2
Solution
To get to the answer you need to have knowledge of the periodic table and its properties. Like, atomic radius and stability of any element increases when we move from top to bottom in the periodic table. Stability means having lower energy and balanced charge and is in equilibrium with the environment.
Complete step by step answer:
Let’s start with understanding superoxides. It is a compound that contains the superoxide ion which has the chemical formula O2− . The name of the anion is dioxide i.e. have negative charge of −1 . This superoxide is reactive oxygen ion as it widely occurs in nature because of the reduction of one electron from dioxygen O2 . Both oxygen and superoxide anion are free radicals that exhibit paramagnetism. The structure of superoxide ion is as given below.
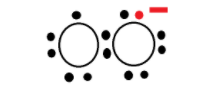
Now, let’s see the compounds given in options.
1. KO2 which is called potassium superoxide is an inorganic compound. It is a yellow coloured paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as CO2 scrubber, H2O dehumidifier and O2 generators in rebreathers, spacecraft, submarines and spacesuit as life support systems. It is produced by burning molten potassium in an atmosphere of oxygen.
K+O2→KO2
This salt consists of K+ and O2− ions linked to each other by ionic bonding.
2. RbO2 which is called Rubidium oxide is a chemical compound which is highly reactive towards water so it cannot be accepted to occur naturally. It is found as an impurity in silicate or aluminosilicate. It is a yellow coloured solid and a strong base. Therefore, it reacts with water to form Rubidium Hydroxide.
3. CsO2 commonly called Caesium oxide is any inorganic compound composed of caesium and oxygen. The binary oxides of caesium are known as Cs11O3 , Cs4O , Cs7O and Cs2O . Both the oxides and suboxides are brightly coloured. They form yellow orange hexagonal crystals. They are used in photocathodes to detect infrared signals in devices such as image intensifiers, photo multipliers and TV camera tubes.
Now, according to periodic table potassium, Rubidium and Caesium are in the first row of periodic table with potassium at the top, rubidium in between and caesium at the bottom. We know that atomic radius increases from top to bottom in the periodic table due to increase in ions and therefore stability also increases. Therefore, the order of stability is KO2<RbO2<CsO2 .
**So, the correct answer is option C.
Note: **
Periodic table trends are very important for solving such questions. Properties can be remembered by understanding and memorizing all the elements of the periodic table along with its number in order.
