Question
Question: The correct order of stability of the following resonating structures is: \[\begin{aligned} & ...
The correct order of stability of the following resonating structures is:
& (I){{H}_{2}}C={{N}^{+}}={{N}^{-}} \\\ & (II){{H}_{2}}{{C}^{+}}-N={{N}^{-}} \\\ & (III){{H}_{2}}{{C}^{-}}-{{N}^{+}}\equiv N \\\ & (IV){{H}_{2}}{{C}^{-}}-N={{N}^{+}} \\\ \end{aligned}$$ A. (I) > (II) > (IV) > (III) B. (I) >(III) > (II) > (IV) C. (II) > (I) > (III) > (IV) D. (III)> (I) > (IV) > (II)Solution
Think about the octet rule and the electronegativities of all the atoms that are involved in the making of the bonds. Check if the octets of all the atoms are complete and determine the stability according to that.
Complete answer:
We know that according to the octet rule, the molecules in which the octets of the atoms are completed are more stable. We will check if the octets of all the atoms in the given resonating structures are complete and assign the stability order according to that. We will also consider the lone pairs on the nitrogen atoms while calculating the number of electrons. Keep in mind that hydrogen atoms will not have an octet but a duplet, so we will not consider those.
- (I)H2C=N+=N−
The detailed structure of this molecule that includes the lone pairs is:
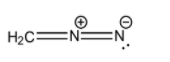
Here, we can see that the octets on all the carbon and nitrogen atoms are satisfied. Carbon has 4 covalent bonds, the central nitrogen has 4 covalent bonds and the terminal nitrogen has 2 covalent bonds, 1 lone pair and a net negative charge.
- (II)H2C+−N=N−
The detailed structure of this molecule that includes the lone pairs is:
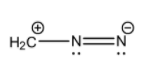
Here, we can see that the octets of both the nitrogen atoms are complete but the orbit of the carbon atom has only 6 electrons from the 3 covalent bonds. In the central nitrogen, it has 3 covalent bonds and 1 lone pair. The terminal nitrogen atom has 2 covalent bonds, 1 lone pair and a net negative charge.
- (III)H2C−−N+≡N
The detailed structure of this molecule that includes the lone pairs is:
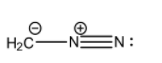
Here, we can see that the octets on all the carbon and nitrogen atoms are satisfied. The carbon atom has 3 covalent bonds and a negative charge, the central nitrogen atom has 4 covalent bonds, and the terminal nitrogen atom has 3 covalent bonds and 1 lone pair.
- (IV)H2C−−N=N+
The detailed structure of this molecule that includes the lone pairs is:
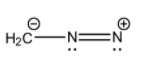
Here, we can see that the octet of the terminal nitrogen is not completed but the other atoms have complete octets. The carbon atom has 3 covalent bonds and a negative charge, the central nitrogen atom has 3 covalent bonds and a lone pair, and the terminal nitrogen atom has 2 covalent bonds and a lone pair which adds up to 6 electrons and not 8.
From the above analysis, we can say that the structures (I) and (III) are more stable than (II) and (IV).
Now we will determine which one of them is more or less stable according to the electronegativities and the charges present on the atoms.
First, let us see which resonating structure among (I) and (III) is more stable:
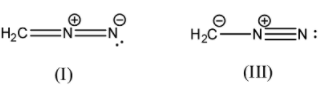
Here we can see that in (I) the charge on the central nitrogen atom is the same so it will not affect the stability. We know that nitrogen is more electronegative than carbon, so a negative charge on the nitrogen atom will be more stable than a negative charge on the carbon atom. So, we can say that (I) is more stable than (III).
Now, let us see which resonating structure among (II) and (IV) is more stable:
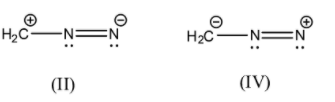
Here too, we will apply the same rule of electronegativity, the charges on the terminal nitrogen and carbon atoms are flipped in both the structures. Nitrogen is more electronegative than carbon, so by the earlier logic, we can say that (II) is more stable than (IV).
Hence, from all this analysis, we can conclude that the correct answer to this question is ‘B. (I) >(III) > (II) > (IV)’ .
Note:
Remember that the negative charge that is present on each of the atoms in the structures represents 2 electrons that are present on the atom and not one. This negative charge has the capability to form a bond that consists of 2 electrons in a resonating structure.
