Question
Question: The correct order of relative stability for the given free radicals is ...
The correct order of relative stability for the given free radicals is
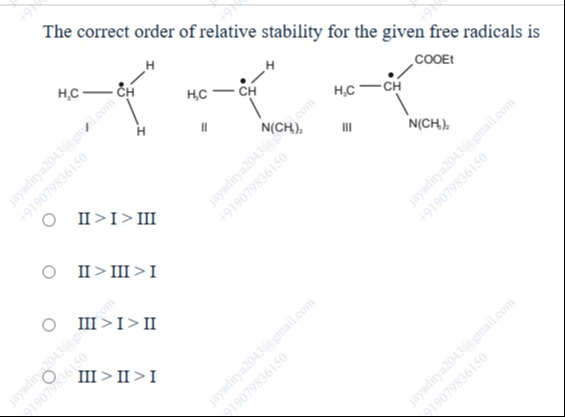
II > I > III
II > III > I
III > I > II
III > II > I
II > III > I
Solution
The stability of free radicals is primarily determined by resonance and hyperconjugation. Electron-donating groups attached to the carbon with the unpaired electron stabilize the radical, while electron-withdrawing groups destabilize it.
Let's analyze the stability of each free radical:
I: H3C−C˙H2 (Ethyl radical). This is a primary free radical. Its stability is due to hyperconjugation from the 3 α-hydrogens on the adjacent methyl group.
II: H3C−C˙H−N(CH3)2. This is a secondary free radical. The carbon atom bearing the radical is adjacent to a nitrogen atom with a lone pair of electrons. This lone pair can be donated through resonance to the electron-deficient radical center, leading to significant stabilization. The resonance structures are:
H3C−C˙H−N(CH3)2↔H3C−CH=N+(CH3)2
Resonance stabilization from an adjacent atom with a lone pair is a very effective stabilizing factor for free radicals.
III: H3C−C˙H−COOEt. This is a secondary free radical. The carbon atom bearing the radical is adjacent to an ethyl ester group. The carbonyl group of the ester is an electron-withdrawing group. However, the radical is on the α-carbon to the carbonyl group, which allows for resonance stabilization:
H3C−C˙H−C(=O)−OEt↔H3C−CH=C−O˙−OEt
This resonance delocalizes the unpaired electron onto the oxygen atom. While oxygen is more electronegative than carbon, the resonance does provide some stabilization. Radicals α to carbonyl groups are generally considered to be resonance stabilized and are more stable than simple alkyl radicals.
Comparing the stability:
- Radical II is strongly stabilized by resonance with the lone pair on nitrogen. This is a very significant stabilizing effect.
- Radical III is stabilized by resonance with the carbonyl group. The extent of stabilization is generally considered to be less than that from an adjacent atom with a lone pair.
- Radical I is a primary alkyl radical, stabilized by hyperconjugation.
Simple secondary alkyl radicals are more stable than primary alkyl radicals due to more hyperconjugation and inductive effect. Radical III is a secondary radical with resonance stabilization from the carbonyl group. Resonance stabilization is typically more significant than hyperconjugation in determining radical stability. Radicals α to carbonyl groups are more stable than simple alkyl radicals.
Therefore, radical II is the most stable due to strong resonance stabilization. Radical III is stabilized by resonance with the carbonyl group, making it more stable than the primary radical I. Radical I is a primary radical, which is less stable than a secondary radical and less stable than radicals stabilized by resonance.
Thus, the order of stability is II > III > I.
