Question
Question: The correct order of hybridization of the central atom in the following species is: \[N{{H}_{3}}\...
The correct order of hybridization of the central atom in the following species is:
NH3,[PtCl4]2−,PCl5andBCl3
a.) dsp2,dsp3sp2,sp3
b.) sp3,dsp2,dsp3,sp2
c.) dsp2,sp2,sp3,dsp3
d.) dsp2,sp3,sp2,dsp3
Solution
Hint: Hybridization of an atom depends on the number of atoms from which the central atom is attached and number of lone pairs attached to it.
Complete step by step solution:
In shortcut form we can calculate hybridization as:
First we have to look at the atom of which we have to calculate hybridization, and count the number of atoms connected to it, not bonds.
And count the number of lone pairs attached to it. And add them both.
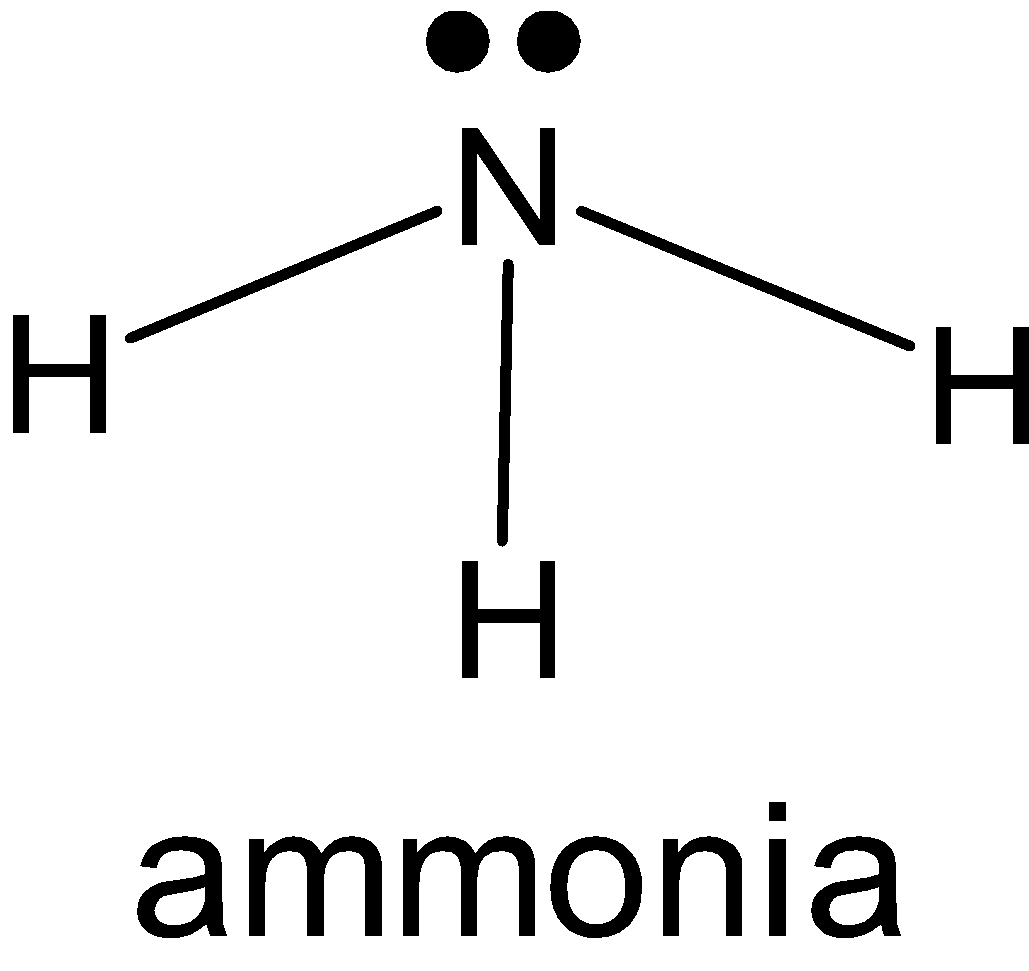
NH3: Nitrogen has 5 electrons in a valence shell from which it makes 3 single bonds with hydrogen and it has one lone pair on it. And NH3has a tetrahedral shape. So, the hybridization of nitrogen is sp3. 3 bond pairs in p-orbital and one lone pair from s-orbital.
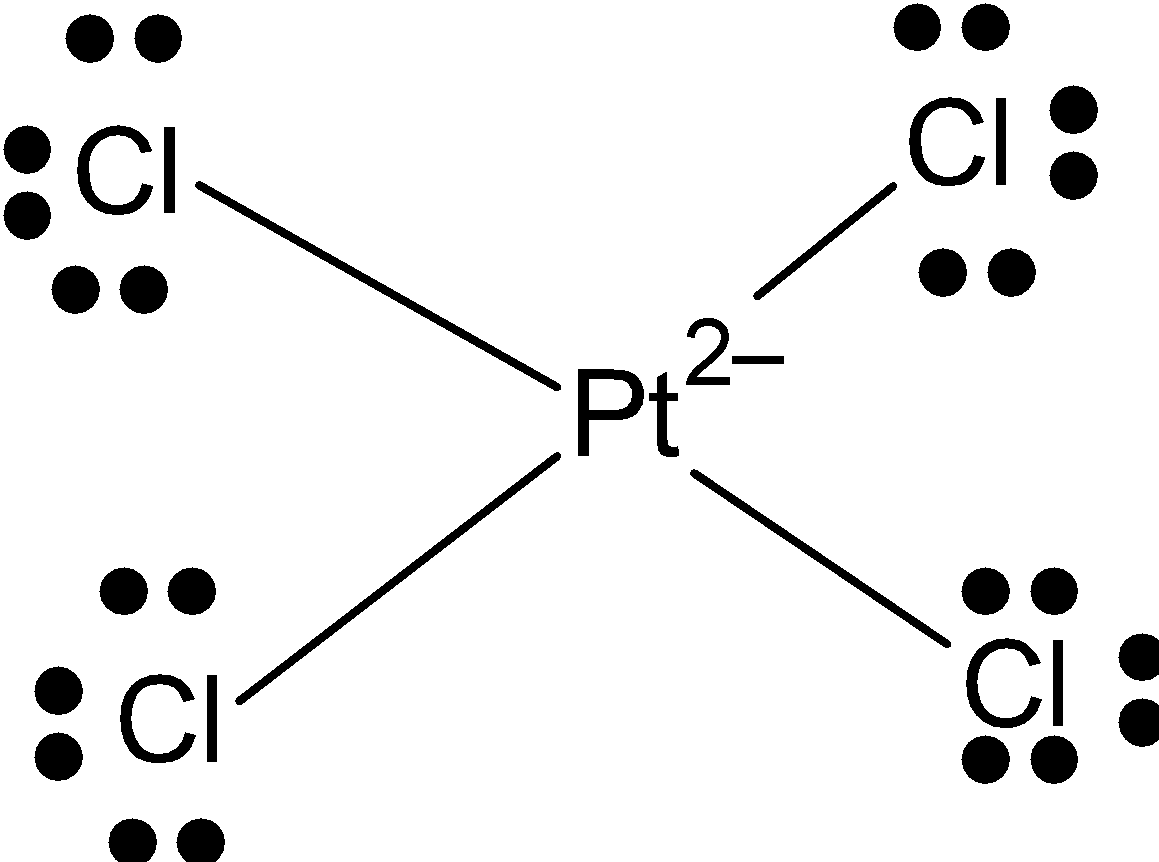
[PtCl4]2−: Pt has 8 valence electrons in valence shell from which it makes 4 bonds with chlorine. And it has a square planar shape. So, the hybridization of Pt+2isdsp2. Because one d-orbital is vacant.
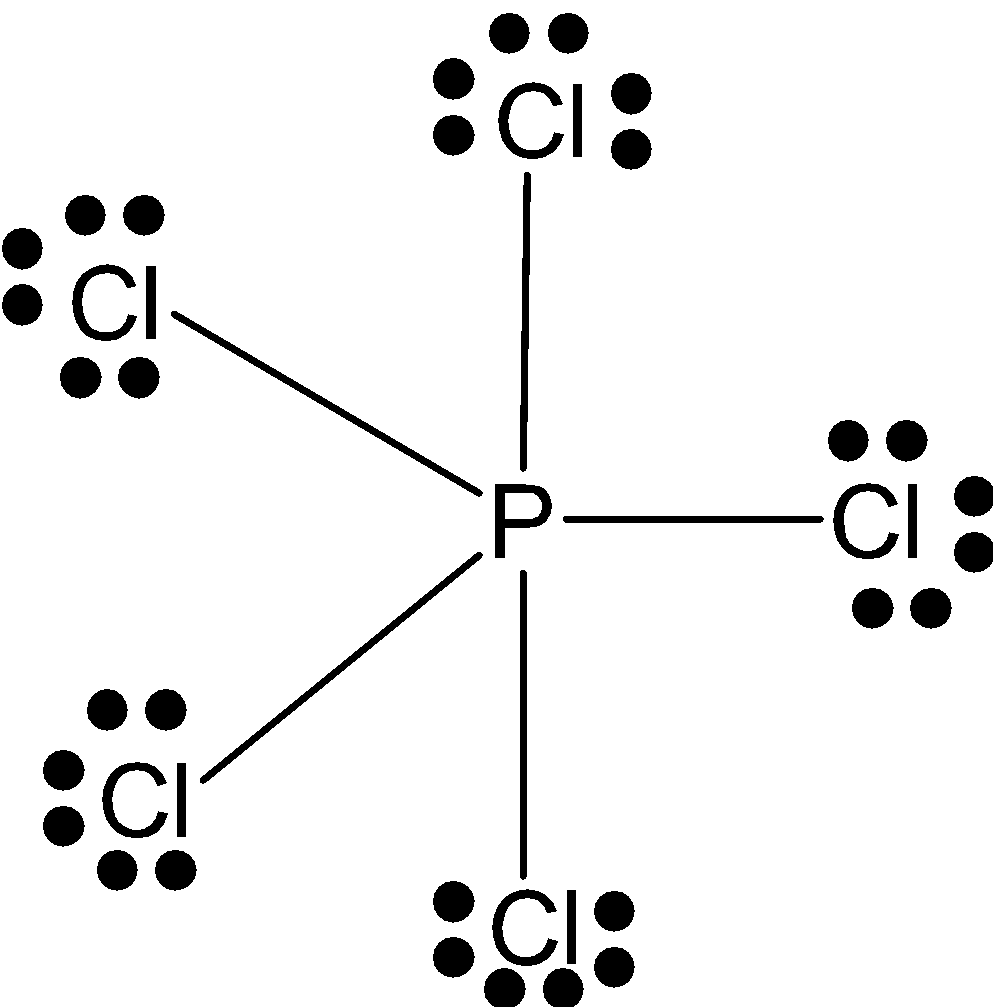
PCl5: P has 5 valence electrons with which it makes 5 bonds with chlorines. And these electron pairs by bonding are filled in one s-orbital 3 in d-orbital and one pair in d-orbital. So, it has sp3dconfiguration and triangular bipyramidal shape.
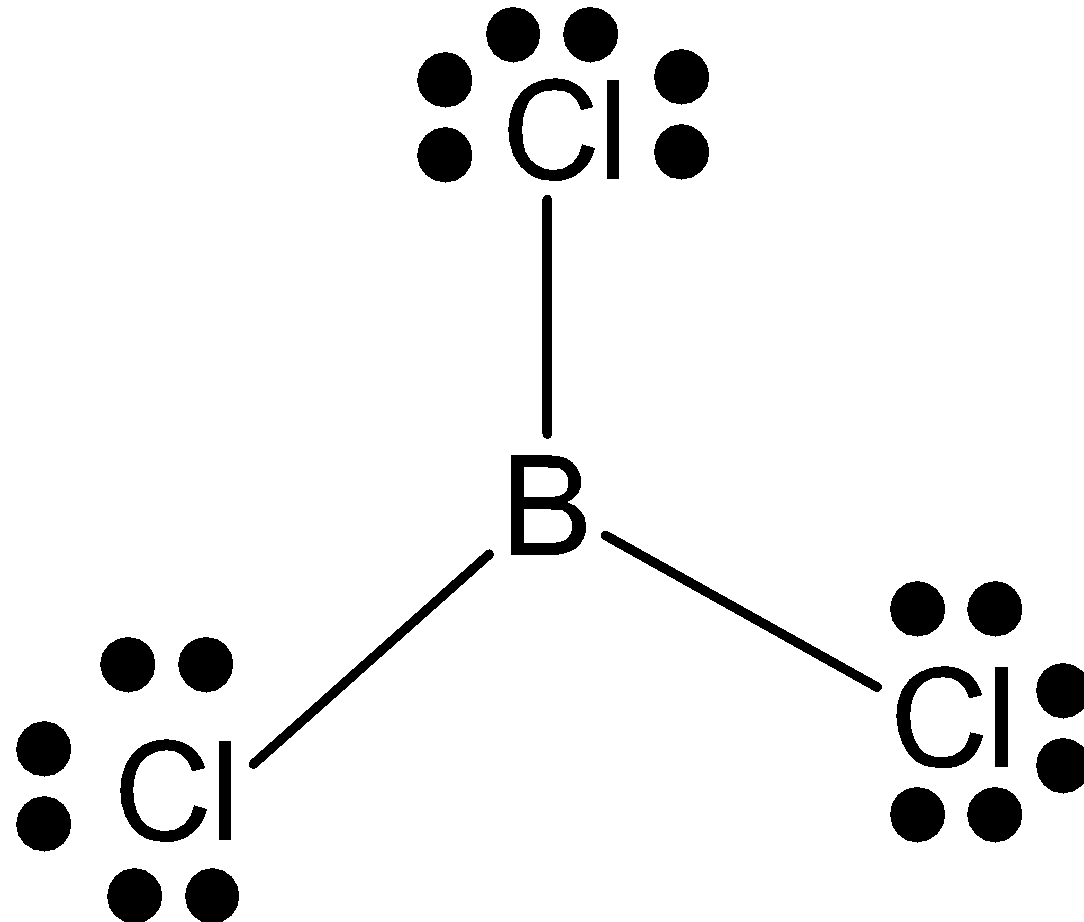
BCl3: B has 3 valence electrons and it makes 3 bonds with chlorine. So, it has hybridization sp2 and triangular shape.
So, the correct answer is “B(sp3,dsp2,dsp3,sp2)”.
Note: [PtCl4]2− is an exception because it has 5d-orbital and here chlorine acts as strong electrons. So, two unpaired electrons of d-orbital get paired so one d-orbital is vacant. But in case of NH3, nitrogen does not have d orbital so all electrons get filled in p orbital.
