Question
Question: The correct order of C-N bond length in the given compound is: P: \(C{{H}_{3}}CN\) Q: HNCO R: \(C{...
The correct order of C-N bond length in the given compound is:
P: CH3CN Q: HNCO R: CH3CONH2
A. P > Q > R
B. P = Q = R
C. R > Q > P
D. R > P > Q
Solution
The length of the bond is going to depend on the type of bond it is. If the bond present in the molecule is a triple bond then the length is very less. The order of length of the different types of bonds is as follows.
Triple bond length < Double bond length < Single bond length.
Complete answer:
- In the given question there are three molecules.
- We have to find the order of C-N length and have to arrange in an order according to their bond length.
- To know about the length of C-N bond in the given molecules, we should draw the structures of the given molecules.
- The structure of P is as follows.
CH3−C≡N
- The structure of Q is as follows.
H−N=C=O
- The structure of R is as follows.
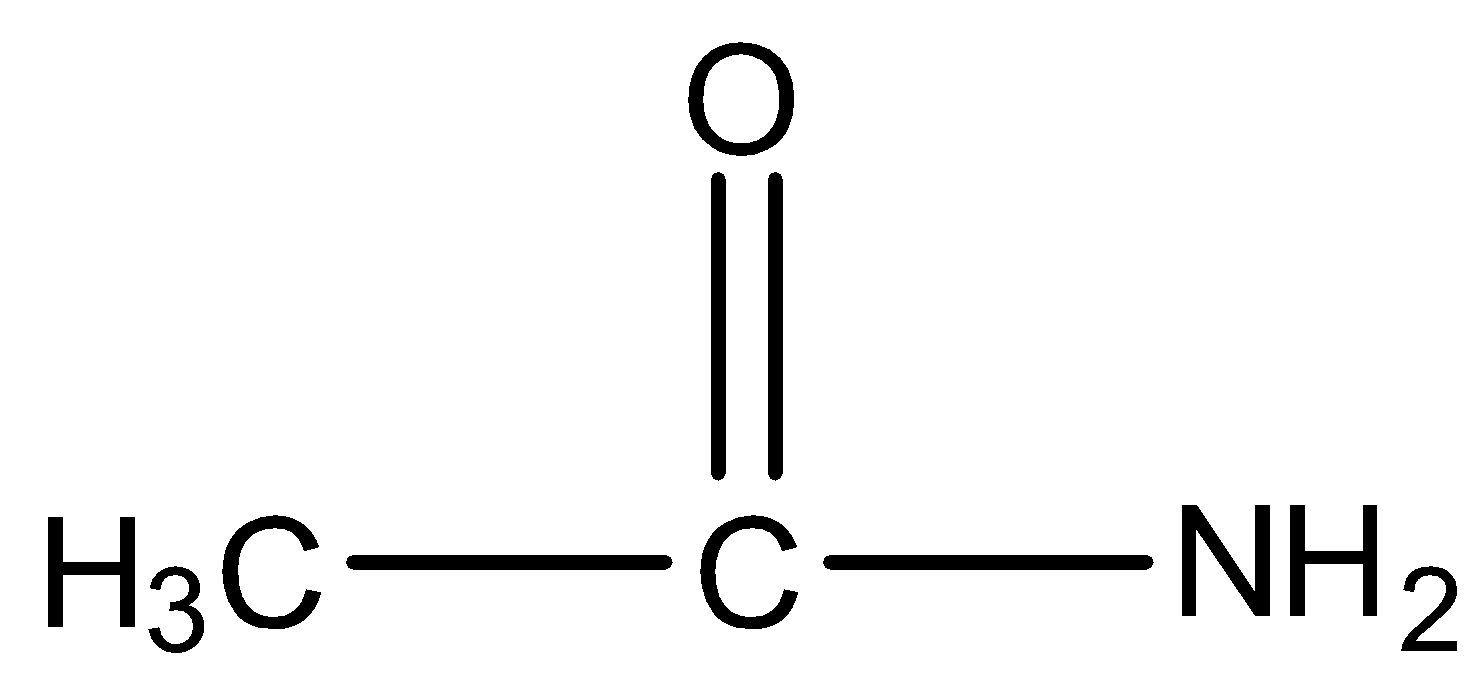
- Molecule P contains a triple bond in between carbon and nitrogen.
- Molecule Q contains a double bond between carbon and nitrogen.
- Molecule R contains a single bond between carbon and nitrogen.
- Therefore the order of C-N bond length in the given molecules is as follows.
R > Q > P
So, the correct option is C.
Note:
We have to check initially the type of bonds present in the given molecules and after knowing about the type of bonds present in the given molecules, we have to arrange them as per bond length. Triple bond always has less bond length when compared to double bond. Double always has less bond length when compared to single bond length.
