Question
Question: The correct order of boiling point of following isomeric amines is: \({C_4}{H_9}N{H_2},{\left( {{C...
The correct order of boiling point of following isomeric amines is:
C4H9NH2,(C2H5)2NH,C2H5N(CH3)2
A.C2H5N(CH3)2>(C2H5)2NH>C4H9NH2
B.(C2H5)2NH>C2H5N(CH3)2>C4H9NH2
C.C4H9NH2>(C2H5)2NH>C2H5N(CH3)2
D.(C2H5)2NH>C4H9NH2>C2H5N(CH3)2
Solution
The following compounds are isomeric amines. The compounds given are primary, secondary and tertiary amines. We will try to figure out the parameter which depends on boiling point and determine the correct order of boiling point.
Complete step-by-step answer: We can observe that the following compounds are isomeric amines as they have identical molecular formula.
First we will draw the chemical structures of the following isomeric amines and then we will be able to identify them as primary, secondary and tertiary.
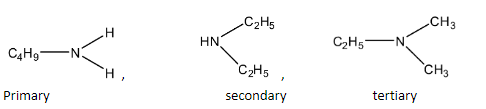
We can easily observe that in primary amine nitrogen is bonded with a single alkyl group and termed as primary amine. In secondary amine nitrogen is bonded with two alkyl groups and termed as secondary amine. In tertiary amine the nitrogen is bonded with three alkyl groups and therefore termed as tertiary amine.
We will introduce one term here intermolecular hydrogen bonding. Intermolecular hydrogen bonding is the bonding in which hydrogen is bonded with the more electronegative element.
And it is also known that more the hydrogen bonding higher the boiling point.
Now we will check hydrogen bonding in primary secondary and tertiary amines. It can be easily observed that intermolecular hydrogen bonding is more in primary amines than in secondary amines. In tertiary amines there is no hydrogen bonding. Therefore, we can conclude that primary>Secondary>tertiary which implies that C4H9NH2>(C2H5)2NH>C2H5N(CH3)2
Therefore, the correct option is (C).
Note: We can conclude that extent of hydrogen bonding is directly proportional to the boiling point. Higher the extent of hydrogen bonding higher the boiling point of a molecule.
There is no hydrogen bonding in tertiary amines as there is no hydrogen attached to the nitrogen atom.
