Question
Question: The correct order of basicity of the above compounds is given. Identify the correct statement (s): ...
The correct order of basicity of the above compounds is given. Identify the correct statement (s):
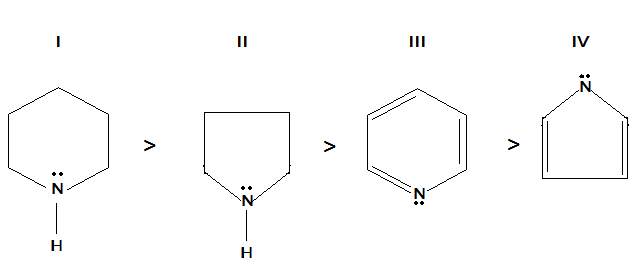
(A) In III, a lone pair of electrons is involved in delocalization but not in II.
(B) In IV, lone pairs of electrons are involved in delocalization but not in III.
(C) I is more basic than II because a six membered ring is more stable than a five membered ring.
(D) In III, lone pair of electrons is present in sp orbital but in I, II and IV lone pair of electrons are present in sp2 orbital.
Solution
Let us first study the given structures and study the delocalization of lone pairs in them. Out of these we have to find out the correct answer on the basis of delocalization since the basicity is given in the correct order of these compounds.
Complete step by step answer:
Here, we know that the lone pair is delocalized because of instability of the lone pair in the ring. In structures I, II and IV have the delocalization in the lone pairs but the lone pair in III does not involve delocalization. The reason is:
Structure III already possesses the stable conjugated system having three double bonds and a lone pair can easily be donated to a hydrogen ion or any Lewis acid.
In structure IV, the lone pair electrons of the nitrogen atom are involved in delocalization to form a conjugated system of pi electrons, leading to greater stability of the ring.
Thus the correct answer is option B.
Note:
Remember that the six membered ring is more basic and stable than the five membered ring. The delocalization of lone pair always depends on the stability of the ring such that if the structure is of pyridine then this is more stable than the pyrrole (five membered ring) and there is no delocalization in pyridine as the structure is stable as compare to pyrrole.
