Question
Question: The correct order of basicity is...
The correct order of basicity is
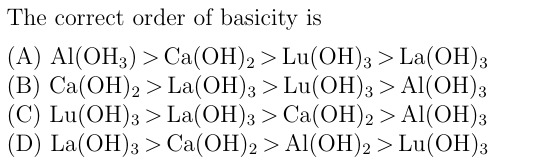
Al(OH3) > Ca(OH)2 > Lu(OH)3 > La(OH)3
Ca(OH)2 > La(OH)3 > Lu(OH)3 > Al(OH)3
Lu(OH)3 > La(OH)3 > Ca(OH)2 > Al(OH)3
La(OH)3 > Ca(OH)2 > Al(OH)2 > Lu(OH)3
B
Solution
To determine the correct order of basicity for the given hydroxides, we need to consider the periodic trends and the properties of the metal ions. Basicity of metal hydroxides M(OH)n depends on the ease with which the M−O bond can break, releasing OH⁻ ions. This ease is favored by:
- Larger ionic size of the metal (M): A larger cation leads to a weaker electrostatic attraction for the hydroxide ion, making the M−O bond more ionic and easier to break.
- Lower charge on the metal ion (M): A lower charge on the cation results in less polarizing power, leading to a more ionic M−O bond and thus higher basicity.
Let's analyze each hydroxide:
- Al(OH)3: Aluminum is in Group 13, Period 3. Al3+ has a small size (53.5 pm) and a high charge (+3). This leads to significant covalent character in the Al−O bond. Al(OH)3 is amphoteric, meaning it can act as both an acid and a base, but it is a very weak base.
- Ca(OH)2: Calcium is an alkaline earth metal in Group 2, Period 4. Ca2+ has a moderate size (100 pm) and a charge of +2. Ca(OH)2 is a strong base, though its solubility is limited.
- La(OH)3: Lanthanum is the first element of the lanthanide series (f-block). La3+ has a relatively large size (103.2 pm) and a charge of +3. La(OH)3 is a strong base.
- Lu(OH)3: Lutetium is the last element of the lanthanide series. Lu3+ has a smaller size (86.1 pm) than La3+ due to lanthanoid contraction, and a charge of +3. Lu(OH)3 is a base, but weaker than La(OH)3.
Now let's compare them:
-
Al(OH)3 vs. other hydroxides: Al(OH)3 is amphoteric and generally considered a very weak base compared to alkaline earth metal hydroxides and lanthanide hydroxides. Therefore, Al(OH)3 will be the least basic.
-
La(OH)3 vs. Lu(OH)3: Both are lanthanide hydroxides. Due to the lanthanoid contraction, the ionic radius decreases from La3+ to Lu3+. A smaller cation has a higher charge density, leading to greater polarizing power and increased covalent character in the M−OH bond. This makes the hydroxide less basic.
Thus, La(OH)3>Lu(OH)3. -
Ca(OH)2 vs. La(OH)3: This is the most crucial comparison.
- Ca2+: Charge +2, ionic radius ≈ 100 pm.
- La3+: Charge +3, ionic radius ≈ 103.2 pm.
While La3+ is slightly larger than Ca2+ (which favors higher basicity for La(OH)3), the higher charge of La3+ (+3 vs +2) significantly increases its polarizing power. A higher charge on the metal ion generally leads to a more covalent M−O bond, making the hydroxide less basic. The effect of charge is usually more dominant than a small difference in size.
Therefore, Ca(OH)2>La(OH)3.
Combining these comparisons, the order of basicity is:
- Ca(OH)2 (most basic among the given)
- La(OH)3 (stronger than Lu(OH)3 due to larger size)
- Lu(OH)3 (weaker than La(OH)3 due to lanthanoid contraction)
- Al(OH)3 (least basic, being amphoteric)
So, the correct order is:
Ca(OH)2>La(OH)3>Lu(OH)3>Al(OH)3
