Question
Question: The correct order of basicity for the following molecules is ...
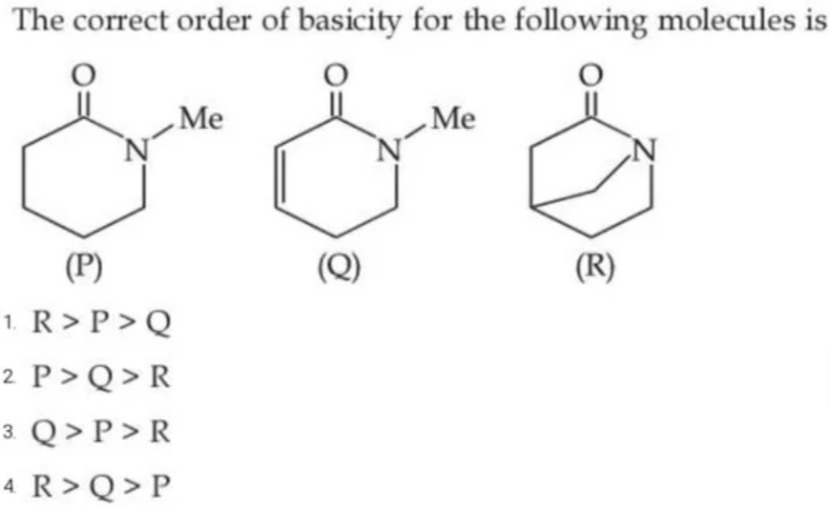
The correct order of basicity for the following molecules is
A
R > P > Q
B
P > Q > R
C
Q > P > R
D
R > Q > P
Answer
R > P > Q
Explanation
Solution
(R): The bridging bond forces the amide into a twisted conformation, reducing effective resonance delocalization of the nitrogen lone pair with the carbonyl. This increases the availability of the lone pair for protonation, making (R) the most basic.
(P): While still an amide, the presence of an N–Me group partially hinders resonance compared to a plain amide, giving it intermediate basicity.
(Q): The extra double bond in the ring extends conjugation with the carbonyl, enhancing resonance delocalization of the nitrogen lone pair. This makes it the least basic.
Thus, the order is: (R) > (P) > (Q).
