Question
Question: The correct order of acidity of alcohols is? A.I > II > III B.III > II > I C.I > III > II D....
The correct order of acidity of alcohols is?
A.I > II > III
B.III > II > I
C.I > III > II
D.II > III > I
Solution
Alcohols are those organic molecules that are formed by linking −OH group to any alkyl or aryl parent chain. This −OH substituent is known as the alcoholic functional group. To put this in simpler terms, alcohols are a homologous series which contain the −OH group. This −OH group is also commonly known as hydroxyl group.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Now, any chemical species is either acidic, basic or neutral in nature. But what do these terms mean?
Basic compounds are those compounds which can readily donate lone pairs of electrons in a chemical reaction. This means that basic compounds are nucleophilic compounds. On the other hand, acidic compounds are those compounds which accept lone pairs of electrons. This means that acidic compounds are electrophilic compounds.
Now moving to the question, the acidic character of an alcohol in alkyl alcohols can be determined by understanding the strength of the conjugate base of the given acid. A conjugate base can be understood as an intermediate product that is formed when an acid tends to gain electrons or lose hydrogen ions (protons). Stronger the acidic character of a species, lower would be the strength of the base. The conjugates of the given alcohols can be given as:
| 1∘ alcohol | 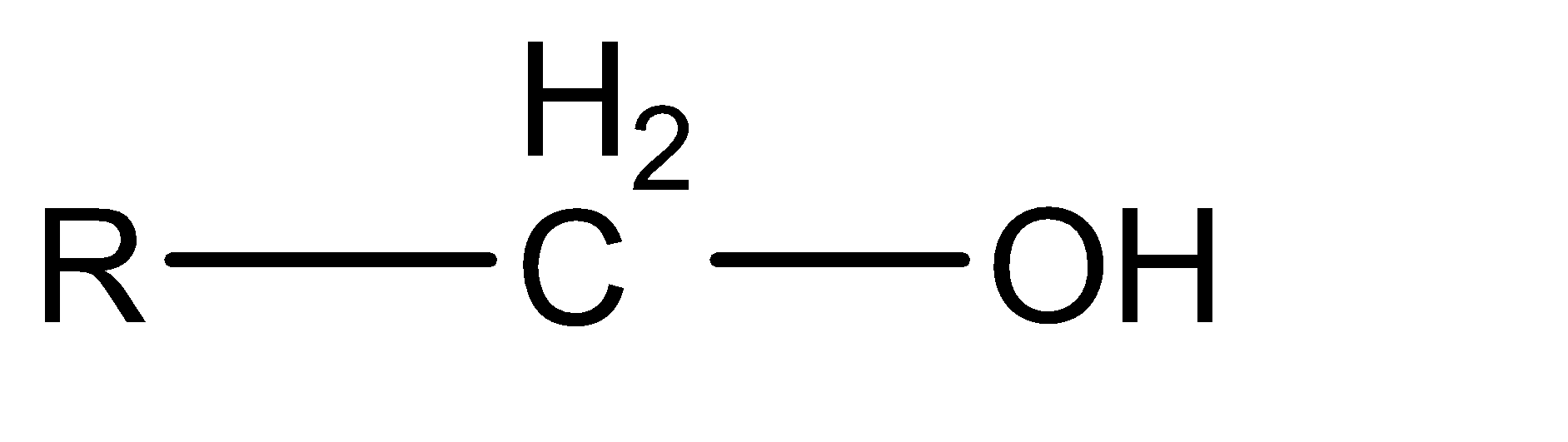 | 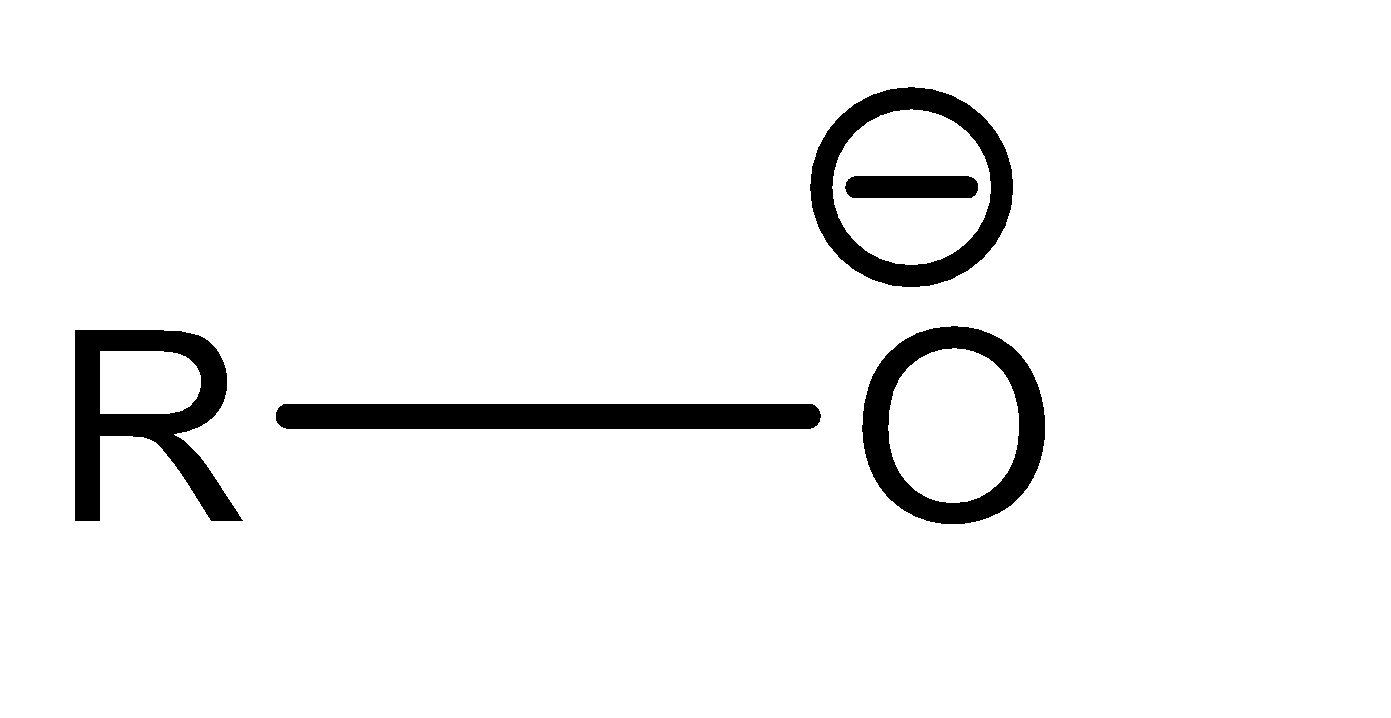 |
|---|---|---|
| 2∘ alcohol | 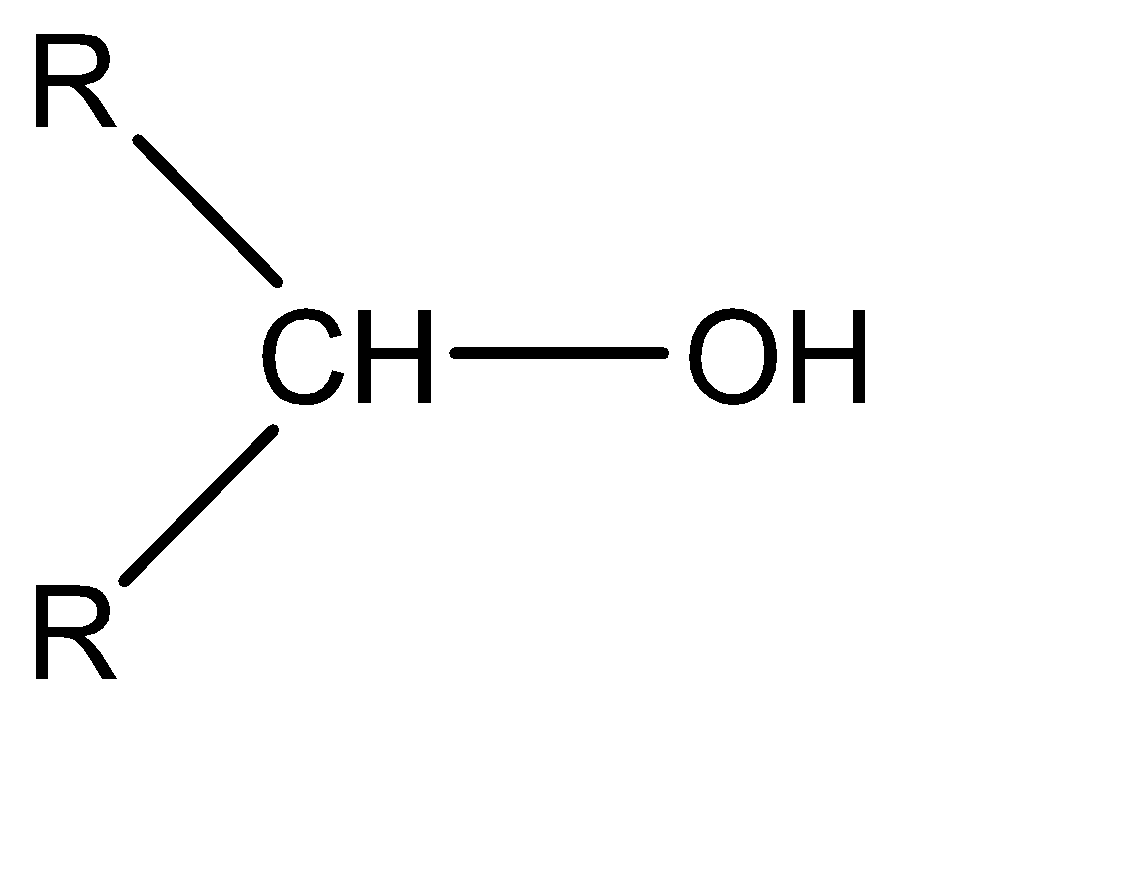 | 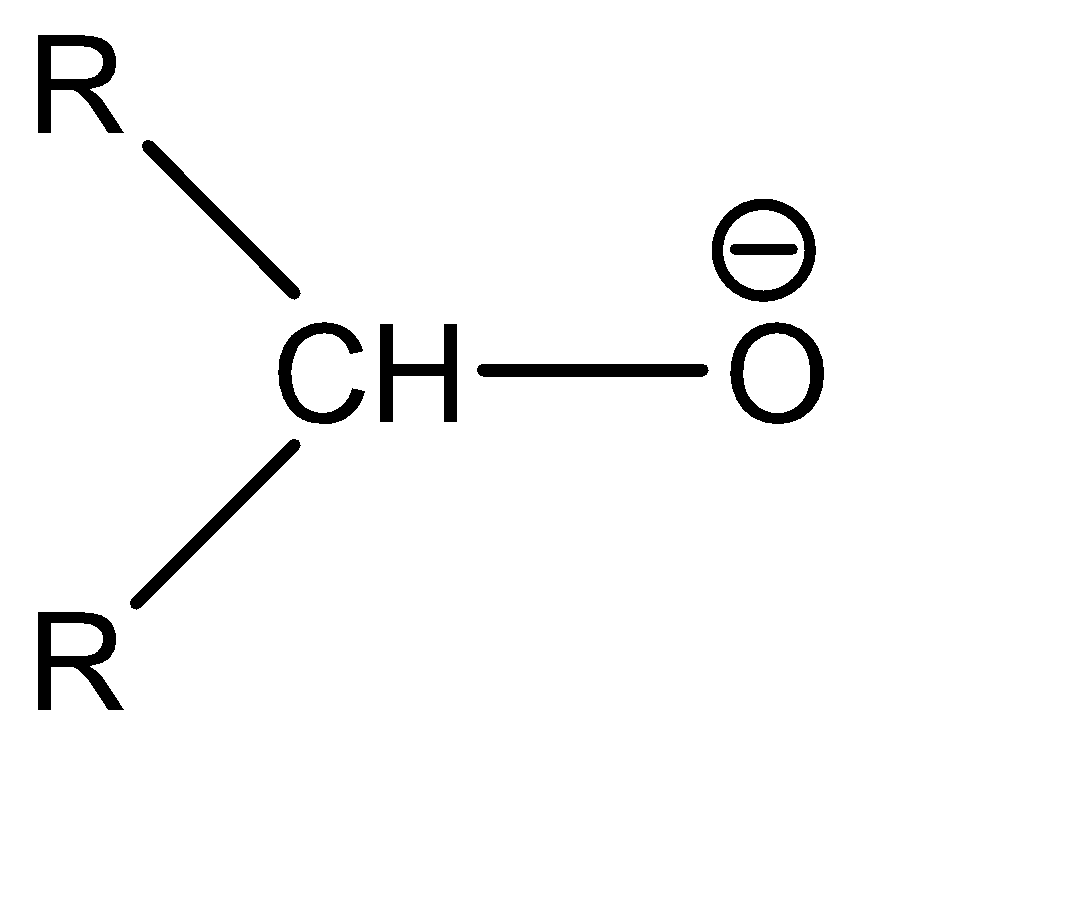 |
| 3∘ alcohol | 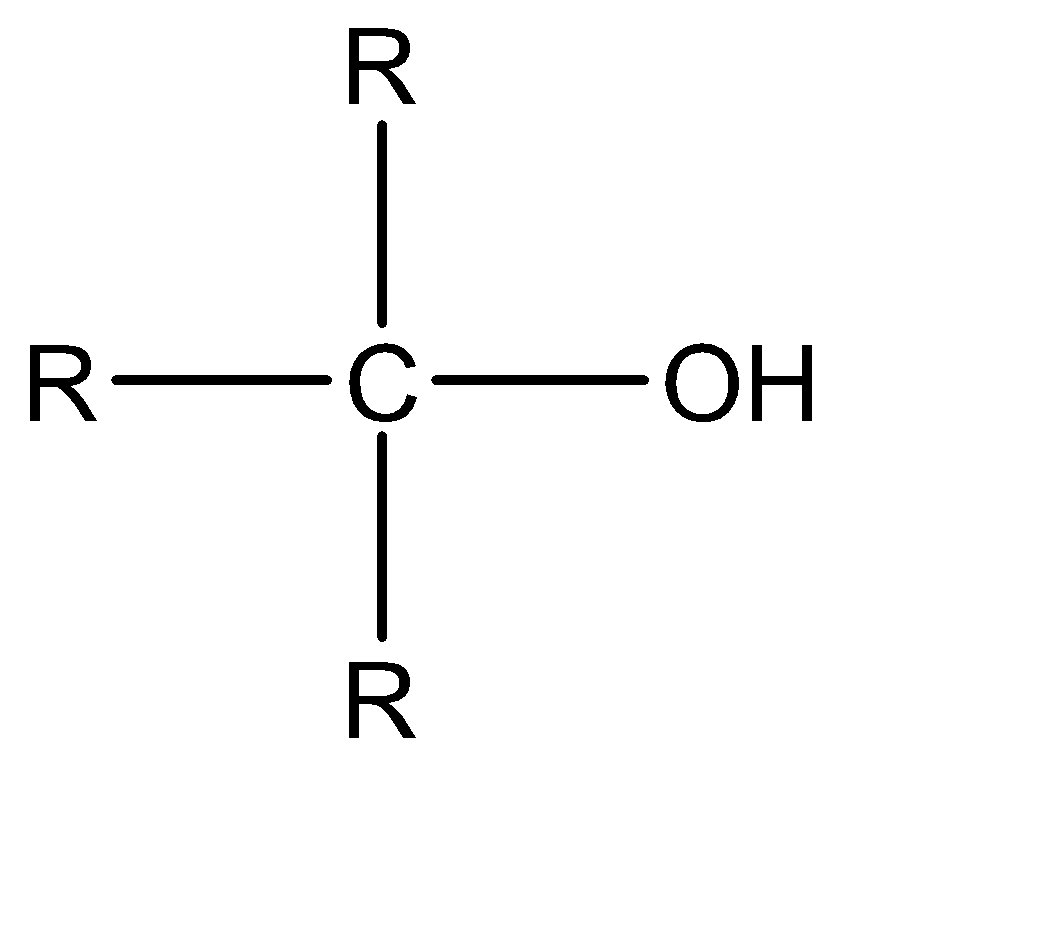 | 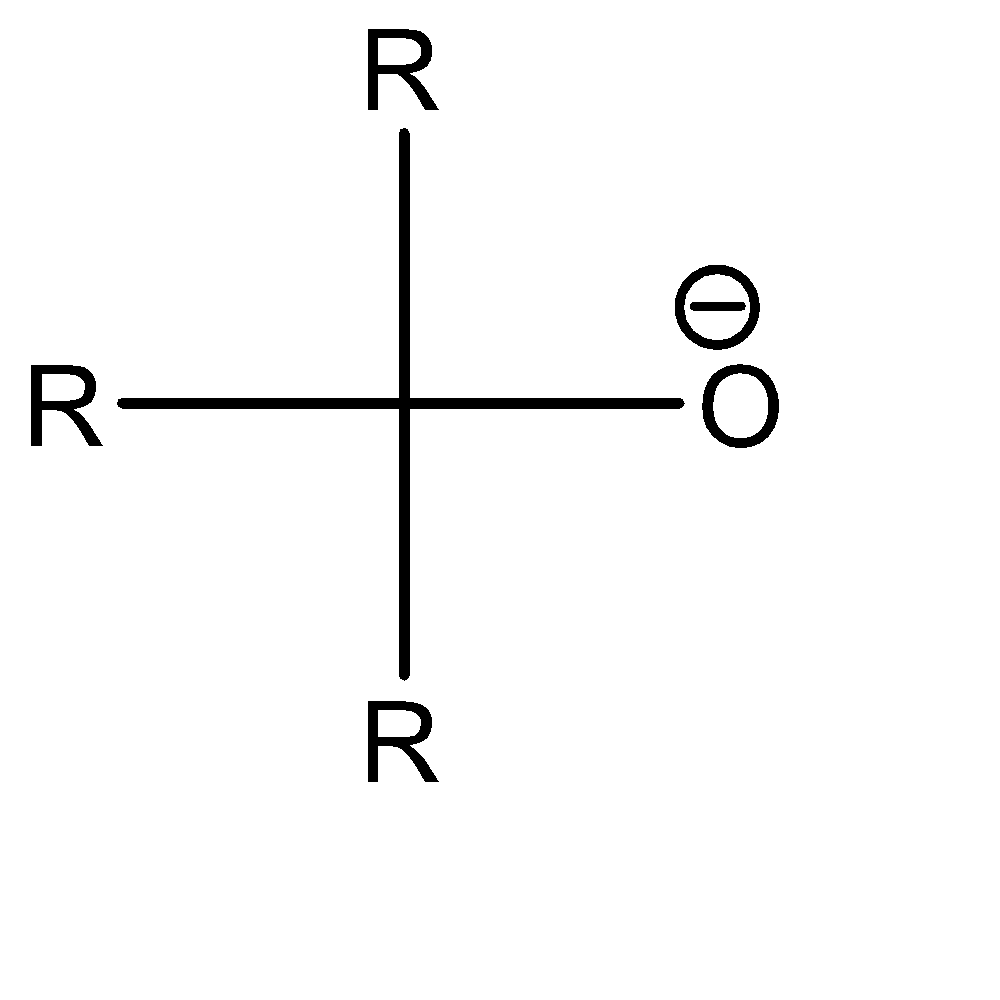 |
An increase in the positive inductive effect of the adjacent substituent atoms increases in the stability of the carbanion. One of the important substituents in a carbanion is the alkyl group. Alkyl group exhibits + I effect and tends to release electrons towards the charged carbon.
Hence the conjugate base of 1∘ alcohol is the weakest while that of 3∘ alcohol is the strongest.
Hence the acidic character of 1∘ alcohol is the greatest while the acidic character of 3∘ alcohol is the smallest.
Hence, Option A is the correct option
Note: Inductive effect: Inductive effect can be understood as an effect that causes unequal sharing of the bonding electron through a chain of atoms due to the electronegative character of certain substituents present in the chain.
