Question
Question: The correct order of acid strength of the above carboxylic acids is 
(I)
(II)
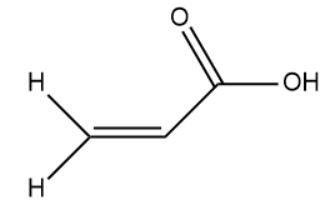
(III)
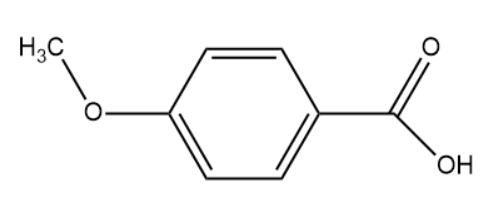
(IV)
(A) I > II > III > IV
(B) II > I > IV > III
(C) I > III > II > IV
(D) III > II > I > IV
Solution
. The acidic strength of the compounds can be determined by the stability of the conjugate base formed. Only when the conjugate base formed is stable, the hydrogen ion will be released easily. The groups attached will contribute to either stabilizing or destabilizing the negative charge after deprotonation. Based on this determine the order of acidic strength of the above compounds.
Complete step by step answer:
Electronic factors are the factor that influence various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
- Inductive effect
- Resonance
- Mesomeric effect
- Electromeric effect
- Hyperconjugation.
The hybridisation of the carbon atom holding the carboxylic group plays a crucial role in determining the acidic strength. The order of strength of acid based on hybridisation is :
sp > sp2 > sp3
We will now determine the hybridisation of the carbon atom holding the carboxylic group.
(I) 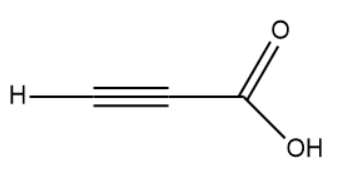
The hybridisation of the carbon atom is sp.
(II) 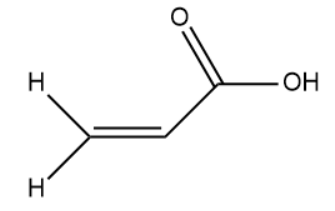
The hybridisation of the carbon atom is sp2.
(III) 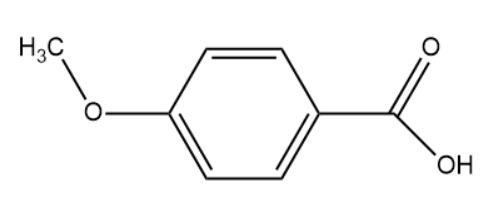
The hybridisation of the carbon atom is sp2.
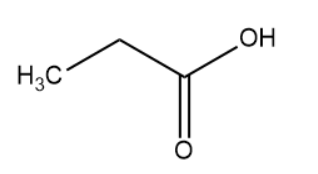
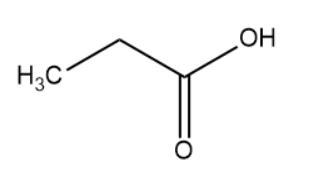
(IV)
The hybridisation of the carbon atom is sp3.
So, the correct answer is “Option A”. I > II > III > IV.
Note: In the above set of compounds, we observe that 2 compounds have the same hybridisation i.e. sp2. However compound (III) has a methoxy group attached which is known to donate electrons. Due to this the conjugate base formed upon deprotonation is very unstable. This is the reason why we consider compound (III) to be less acidic than compound (II) although they possess the same hybridisation.
