Question
Question: The correct option(s) regarding the complex \({[Co(en){(N{H_3})_3}({H_2}O)]^{3 + }}\) \((en = {H_2}N...
The correct option(s) regarding the complex [Co(en)(NH3)3(H2O)]3+ (en=H2N−CH2−CH2−NH2) is/are:
(The question has multiple correct options)
A. It has two geometrical isomers.
B. It will have three geometrical isomers if Bidentate ′en′ is replaced by two cyanide ligands.
C. It is paramagnetic
D. It absorbs light at a longer wavelength as compared to [Co(en)(NH3)4]3+.
Solution
The geometrical isomers are the compounds which are found in heteroleptic complexes having different groups attached to the central metal atom and have different possible geometrical arrangements of the ligands. When two identical groups occupy adjacent positions, the isomer is called cis and when arranged opposite to one another, the isomer is called trans. This also has another type of configuration named as fac/mer isomerism which also comes under the category of geometrical isomerism.
Complete step by step answer: Let us discuss each of the options one by one.
-The structures of the two isomers of the complex are as follows:
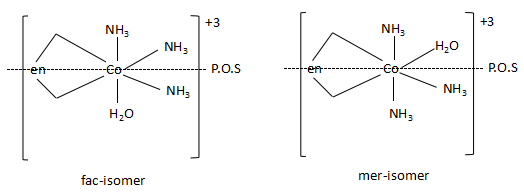
As the complex has two isomers namely, facial and meridional isomers, the statement is correct. The complex has two geometrical isomers. As there are planes of symmetry present in the two isomers, both the complexes are optically inactive.
-When the bidentate ligand, ethylene diamine, is replaced by two cyanide ions, the complex forms three geometrical isomers. They are as follows:
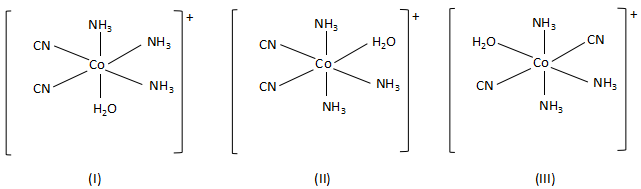
Thus, this statement is also true.
(iii) The magnetic nature of a central metal ion is decided by the presence of unpaired electrons in the orbitals and the surrounding ligands. If the ligands are strong fields, they will pair the electrons and thus the complex will be diamagnetic in nature. If there are unpaired electrons in the complex, it will act as paramagnetic.
In this case, the electronic configuration of cobalt in its ground state is:
Co=[Ar]4s23d7
The electronic configuration of Co3+ ion will be:
Co=[Ar]4s03d6
The surrounding ligands are strong field ligands in the complex. Thus, they will pair all the unpaired electrons and the complex will become diamagnetic. Due to this pairing, the hybridization of the complex will be d2sp3 and the complex will be a low spin complex.
Thus, this statement is wrong.
(iv) It absorbs light at a longer wavelength as compared to [Co(en)(NH3)4]3+. This statement is wrong because in the presence of a strong field ligand, the pairing energy increases and becomes more than that of the crystal field splitting energy. Thus, the wavelength decreases or becomes shorter as the energy gap between t2g and eg orbitals increases.
Thus, the correct options are:
A. It has two geometrical isomers.
B. It will have three geometrical isomers if Bidentate ′en′ is replaced by two cyanide ligands.
Note: The elements of symmetry play a crucial role in deciding the geometry of the complexes in coordination chemistry. There are basically three elements of symmetry. They are planes of symmetry, center of symmetry and axis of symmetry. If any of these elements of symmetry are found in the complexes, they tend to become optically inactive and cannot rotate a plane polarized light.
