Question
Question: The correct IUPAC name for the structure 
is:
A.3−isopropyl−4−methylhexane
B.4−isopropyl−3−methylhexane
C.3−ethyl−2,4−dimethylhexane
D.2−ethyl−3−isopropylpentane
Solution
In synthetic terminology, a favored IUPAC name is a special name, doled out to a compound substance and liked among the potential names produced by IUPAC classification. The favored IUPAC terminology gives a bunch of rules for picking between various conceivable outcomes in circumstances where it is imperative to settle on an extraordinary name.
Complete answer:
The given structure has to be drawn below,
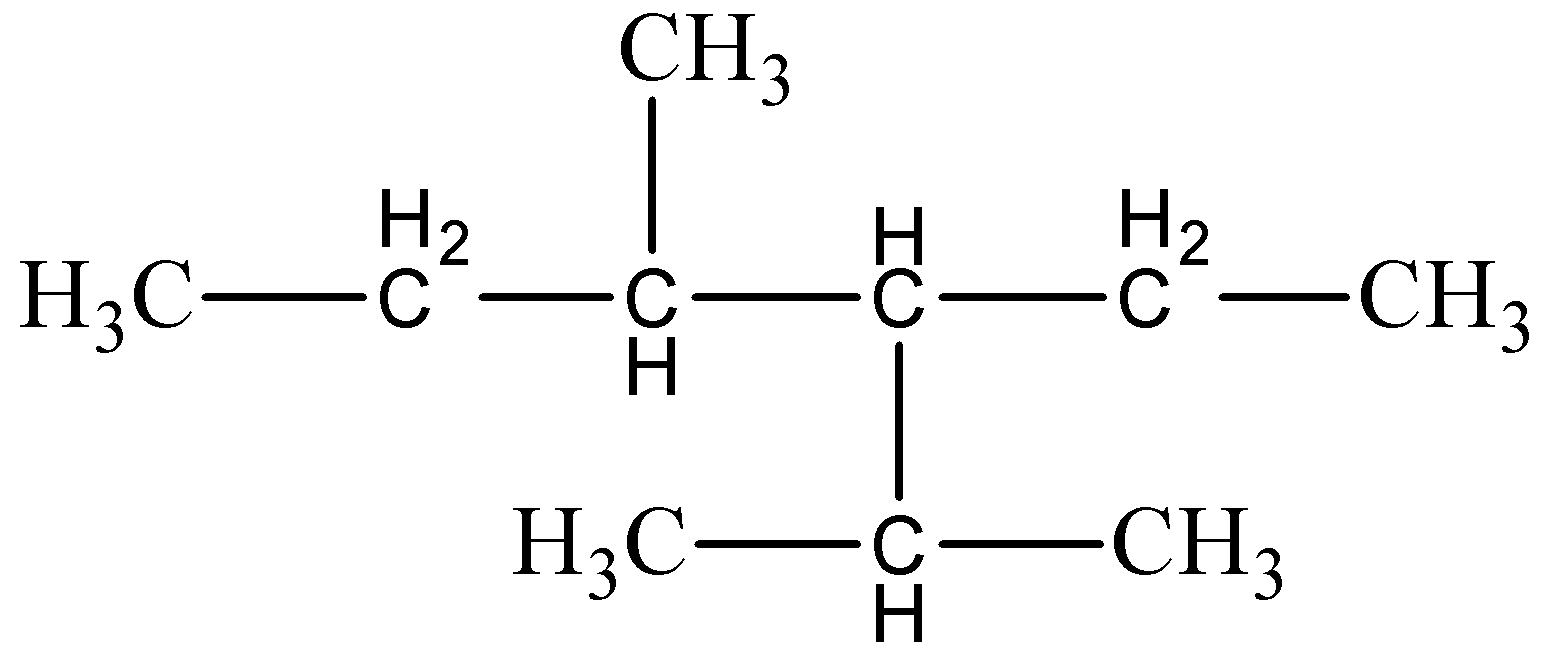
For 3 -isopropyl- 4 -methylhexane,
The structure of the 3 -isopropyl- 4 -methylhexane is given below,
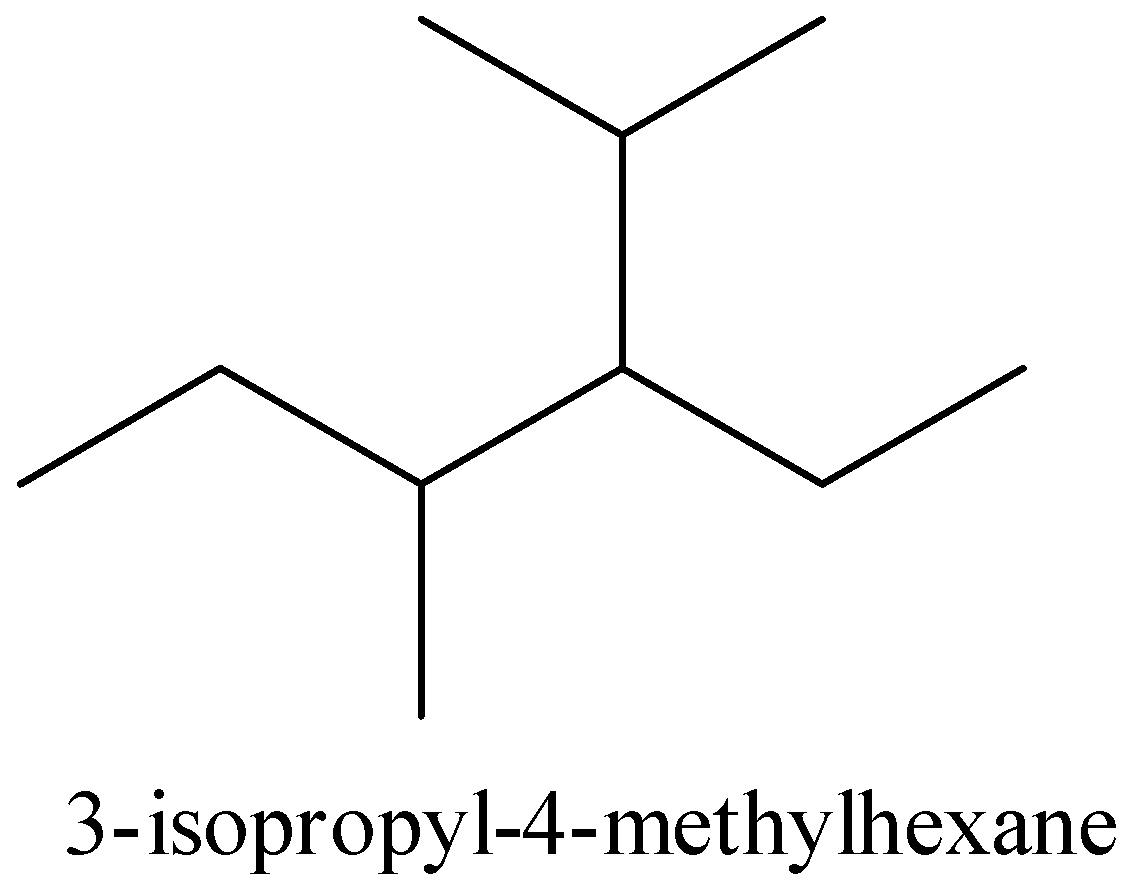
In the given structure and the above structure are not the same. So, that compound is incorrect.
Therefore, option (A) is incorrect.
For 4 -isopropyl- 3 -methylhexane,
The structure of the 4 -isopropyl- 3 -methylhexane is given below,
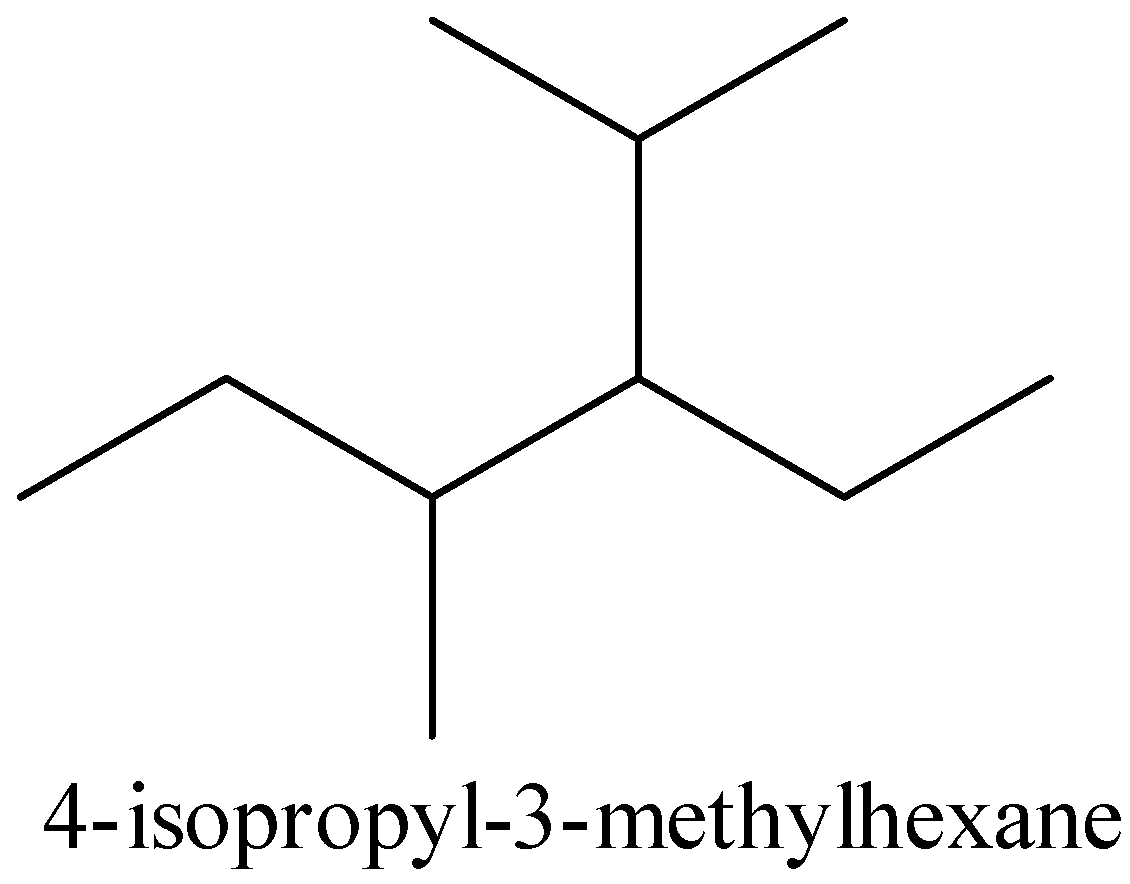
The given structure and the above structure are not the same. So that compound is incorrect.
Therefore, option (B) is incorrect.
For 3 -ethyl- 2 , 4 -dimethylhexane,
The structure of the 3 -ethyl- 2 , 4 -dimethylhexane is given below,
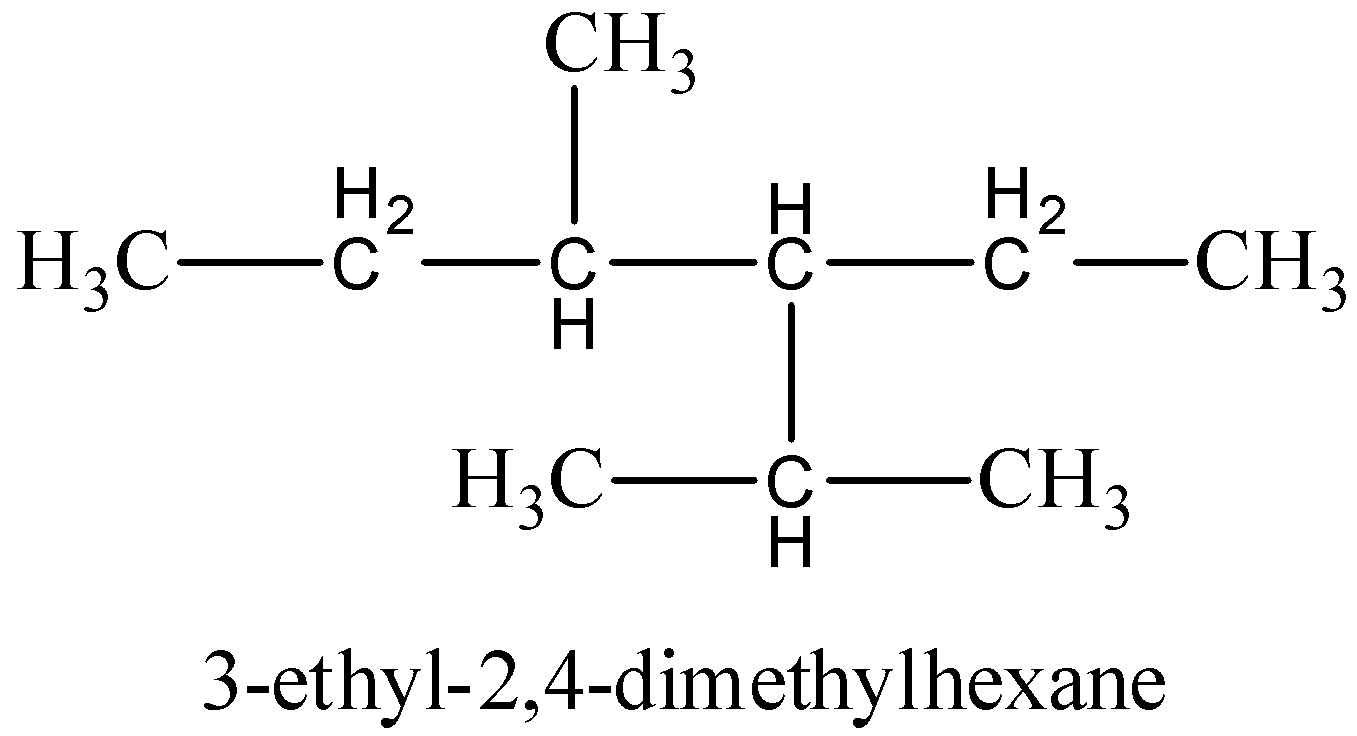
In the given structure and the above structure are the same. So that this compound name is correct. Hence, the IUPAC name of the given compound is 3 -ethyl- 2 , 4 -dimethylhexane, because ethyl group is present in the third carbon and two methyl groups are present in the second and fourth carbon. Then, the carbon skeleton is hexane.
Therefore, option (C) is correct.
For 2 -ethyl- 3 -isopropylpentane,
The structure of 2 -ethyl- 3 -isopropylpentane is given below,
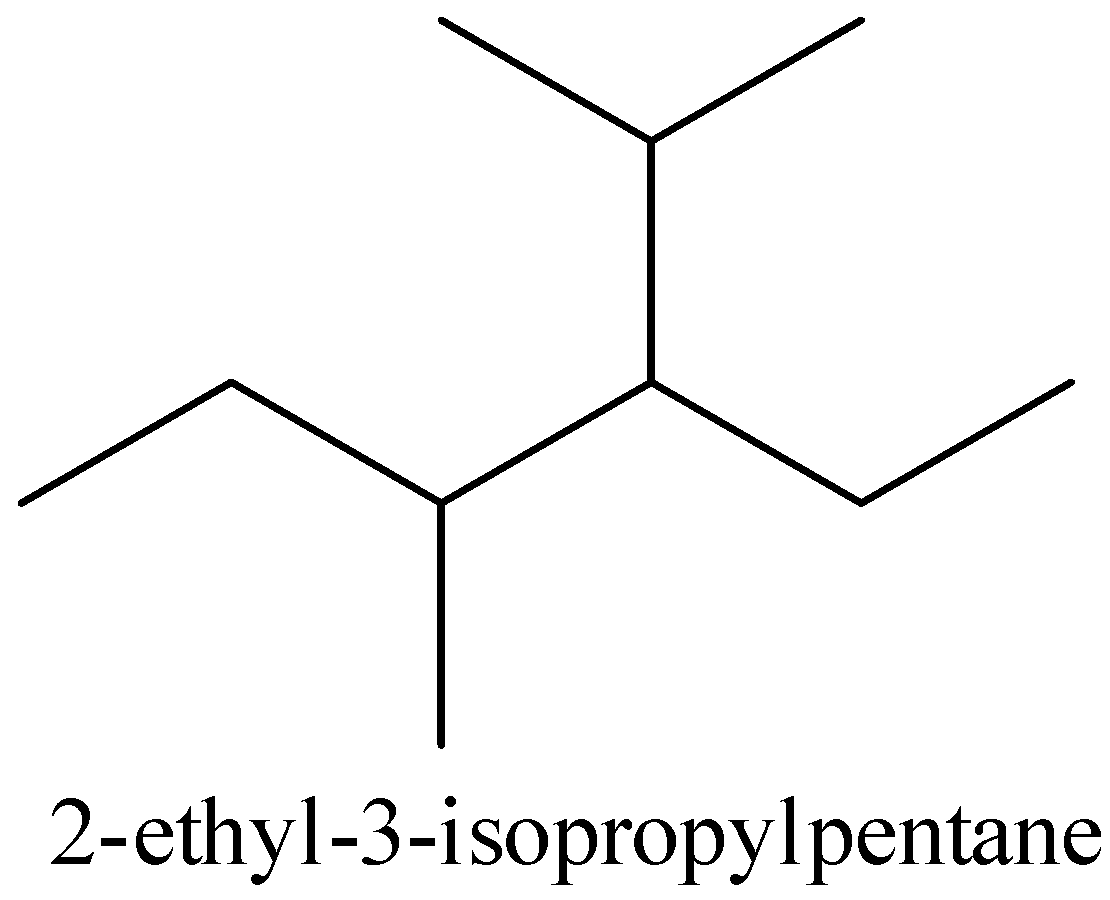
The given structure and the above structure are not the same. So that compound is incorrect.
Therefore, option (D) is incorrect.
Note:
We have to know that in IUPAC classification all mixtures containing carbon iotas are considered as natural mixtures. Natural terminology just applies to natural mixtures containing components from the Groups 13 through 17 . Organometallic mixtures of the Groups 1 through 12 are not covered by natural terminology.
