Question
Question: The correct increasing order of reactivity for the following molecules towards electrophilic aromati...
The correct increasing order of reactivity for the following molecules towards electrophilic aromatic substitution is:
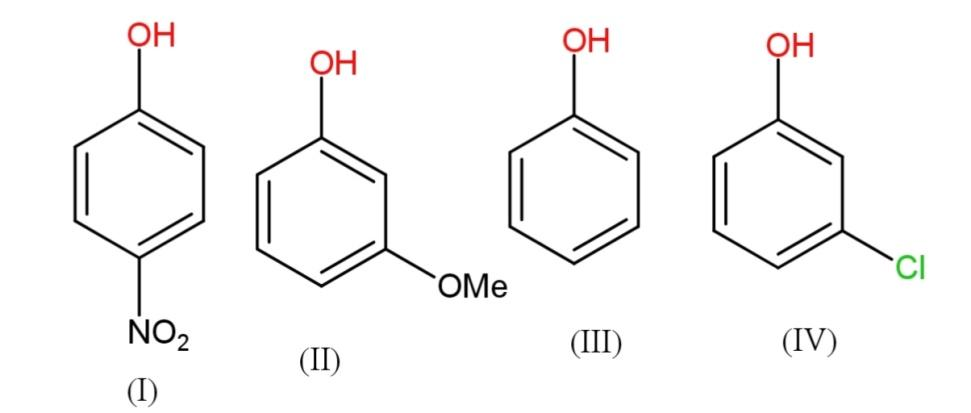
A.I<IV<II<III
B. I<IV<III<II
C. I<III<II<IV
D. I<III<IV<II
Solution
An electrophile is an electron deficient species and readily reacts with electron dense molecules. Electrophilic aromatic substitution involves an electrophile removing a substituted group of atoms and taking its place. The electrophilic aromatic substitution reactions occur where there is greater electron density.
Complete answer:
An electrophilic aromatic substitution is a substitution reaction where the electrophile attaches on the reacting compound removing the already substituted group. An electrophile is the electron loving species that carry a neutral or a positive charge and are considered electron deficient. Therefore electrophilic substitution reaction occurs when there is more electron density on the reacting compounds.
The factor that affects the electron density on compounds is the inductive effect. The −I effect or the negative inductive effect is responsible for withdrawing electrons from any compound as they consist of electronegative atoms that withdraw the electrons. This reduces the electron density. While, a+Ieffect or positive inductive effect is responsible for donating electrons and increases the electron density on the molecule.
Some −I groups are NO2,Cl−,COOH,Br−,etc. while +I groups are methyl, ethyl and other alkyl and alkoxy groups. Thus, −Igroups will have less reactivity in electrophilic aromatic substitution, therefore the increasing order will have nitrophenol < meta - chlorophenol < phenol < methoxy phenol.
Hence the increasing order of reactivity towards electrophilic aromatic substitution is I<IV<III<II.
So option B is correct.
Note:
The order of −Ior negative inductive effect is NO2>Cl, therefore nitrophenol has the least reactivity that chloro phenol in electrophilic aromatic substitution. The positive inductive effect is permanent. The negative inductive effect contains molecules with electronegativity difference therefore they create a negative and positive charge on the molecule affecting the electron density.
