Question
Question: The correct increasing bond angle among \(B{{F}_{3}}\), \(P{{F}_{3}}\) and \(Cl{{F}_{3}}\) follows t...
The correct increasing bond angle among BF3, PF3 and ClF3 follows the order:
A. 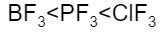
B. 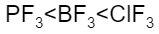
C. 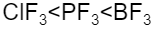
D. 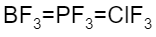
Solution
To solve this question, think about the hybridisation, three-dimensional orientation, and number of lone pairs of electrons on each central atom of the molecule to identify the basic bond angle of the structure and then move on to how lone pairs will affect the bond angle. Then arrange the molecule in increasing order of their bond angle.
Complete step by step solution:
First of all, let us look at the geometry, hybridisation and number of lone pair of electrons of all the molecules given:
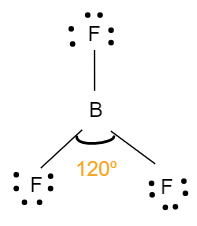
- The geometry of BF3 is trigonal planar. It involves four atoms i.e. one boron atom bonded with three fluorine atoms. It has no lone pairs of electrons on its central atom and has a sp2 hybridisation. This means that the bond angle between the atoms will be 120o, where all the atoms are in one plane. Each one of them also makes an equilateral triangle. The following figure shows the structure and bond angle of the fluoride:
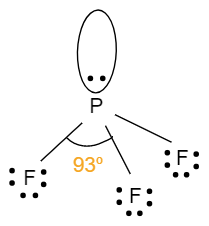
- The geometry of PF3is tetrahedral. It involves four atoms i.e. one phosphorus atom bonded with three fluorine atoms. It has one lone pair of electrons with a hybridisation of sp3.This means that, the bond angle will be less than 109o.The structure with bond angle is shown below:
- The geometry of ClF3 is trigonal bipyramidal. It also involves four atoms in its molecule i.e. one chlorine atom which is bonded with three fluorine atoms. It has two lone pairs of electrons with a hybridisation of sp3d. Due to the presence of lone pairs of electrons, it will have a bond angle less than 90o. So, the structure with bond angle is shown below:
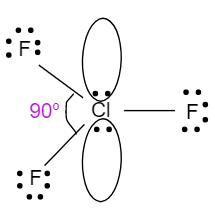
ClF3 with 90o will be less than PF3 with 93o , which will be less than BF3 with bond angle 120o.
Hence, the correct option is C.
Note: Lone pairs are in the orbitals that are shorter and rounder than the orbitals that the bonding pairs occupy. Because of this, there is more repulsion between a lone pair and a bond pair electron thereby forcingly reducing the bond angle. More is the number of lone pairs of electrons, less will be the bond angle of the corresponding molecule.
