Question
Question: The correct graph representing the relation between energy\((E)\)of photoelectrons and frequency \((...
The correct graph representing the relation between energy(E)of photoelectrons and frequency (v)of incident light.
Solution
Hint: When light falls on a metal plate, electrons are ejected from the metal surface with some energy. For finding the right curve between the energy of photoelectrons and the frequency of incident light, we will use Einstein’s equation for the photoelectric emission.
Formula used:
E=hv−W
Complete step by step answer:
The photoelectric effect is described as the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons being emitted in this manner are called photoelectrons. The photoelectric effect is often defined as the ejection of electrons from a metal plate when light falls on it.
The Einstein’s equation for photoelectric effect is given by,
E=hv−W
Where,
E is the energy associated with the photoelectrons
h is the Planck's constant
v is the frequency of incident light
W is the work function of the metal
From above equation, we get,
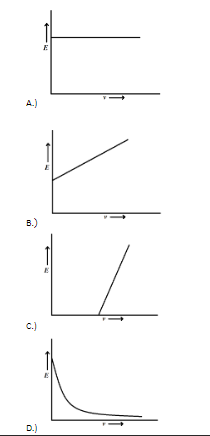
Energy associated with the photoelectrons depends linearly on the frequency of incident light.
The graph between energy (E) of photoelectrons and frequency (v) of incident light will be a straight line with negative slope equal to W.
Hence, the correct option is C.
Additional information:
The photoelectric effect finds its application in the following areas:
Photomultipliers – These are extremely light-sensitive vacuum tubes with a photocathode coated onto part of the inside of the envelope.
Image sensors – Video camera tubes use photoelectric effect to transform an optical image into a scanned electronic signal.
Gold-leaf electroscope – These are designed in order to detect static electricity. Charge being placed on the metal cap spreads to the stem and leaf of the electroscope, which causes the leaf to bend away from the stem.
Moon dust – Light from the Sun hitting lunar dust causes it to become positively charged from the photoelectric effect. The charge dust then repels itself and lifts off the surface of the Moon by electrostatic levitation.
Note:
The amount of energy associated with the emitted photons is directly proportional to the photon’s electromagnetic frequency, or the frequency of incident light, and inversely proportional to the wavelength of incident light. The higher the frequency of the wave, the higher the energy associated with the photon. The longer the wavelength, the lower is the energy associated with the photon.
