Question
Question: The correct decreasing order of reactivity for a given alkyl(R) group in both \({S_N}1\) and \({S_N}...
The correct decreasing order of reactivity for a given alkyl(R) group in both SN1 and SN2 reaction mechanisms is:
A. R−I>R−Br>R−Cl>R−F
B. R−I>R−Cl>R−Br>R−F
C. R−F>R−Cl>R−Br>R−I
D. R−F>RI>R−Cl>R−Br
E. R−Br>R−I>R−F>R−Cl
Solution
Basically, with increase in the size of the halogen atom, the bond length also increases or we can say that the bond strength decreases. Thus, it is very clear that the bond strength decreases. So, by knowing the trends we can easily solve this question.
Complete step by step answer:
Basically, there are two types of for haloalkanes i.e. SN1 and SN2 reactions. First of all, we will discuss the SN1 reaction. It is a substitution nucleophilic unimolecular reaction and is generally a two-step reaction. So, in the first step the carbon-halogen bond breaks heterolytically with the halogen retaining the previously shared pair of electrons and in the second step, the nucleophile reacts rapidly with the carbocation that was formed in the previous step. This reaction follows the first order kinetics. The reaction is as shown:
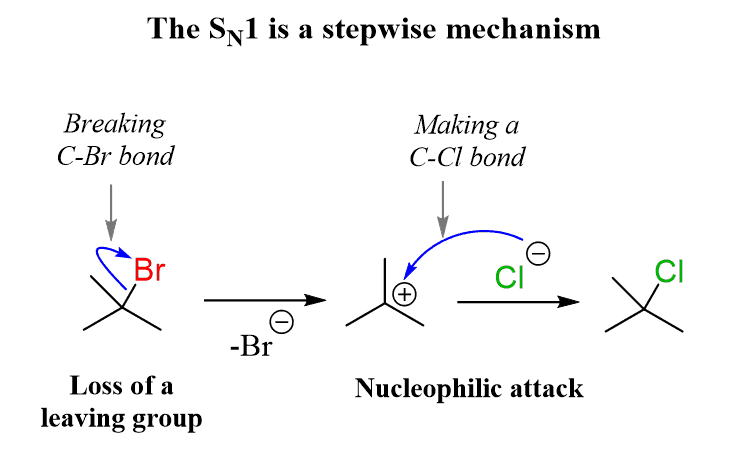
Now, SN2 reaction follows the second order kinetics and the rate of reaction depends upon both halo alkane and the participating nucleophile. So, this reaction is known as substitution nucleophilic bimolecular reaction. It is a one- step reaction and in this reaction the nucleophile attacks the positively charged carbon and the halogen leaves the group. The reaction is as shown:
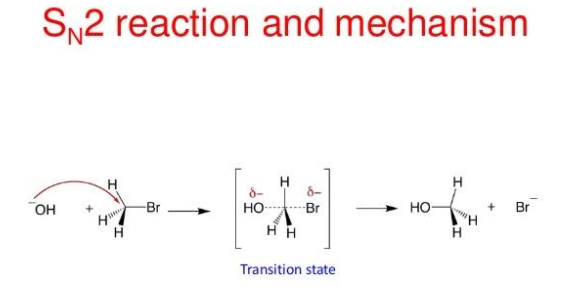
Now, as the size of the halogen atom increases, the bond length also increases or we can say that the bond strength decreases. Thus, the rate of reaction in SN1 and SN2 reaction increases. So, the order of size of halogen atom is:
I>Br>Cl>F
Therefore, the order of reactivity is :
R−I>R−Br>R−Cl>R−F
Hence, option A is correct.
Note: The nucleophilic substitution reaction depends on a number of factors. Some important factors include effect of the solvent, effect of nucleophile, effect of leaving group and the effect of the structure of the substrate.
