Question
Question: The correct combination of names for isomeric alcohols with molecular formula \({C_4}{H_{10}}O\) is/...
The correct combination of names for isomeric alcohols with molecular formula C4H10O is/are :
a.) tert-butanol and 2-methylpropan-2-ol
b.) tert-butanol and 1,1-dimethyl ethan-1-ol
c.) n-butanol and butan-1-ol
d.) isobutyl alcohol and 2-methylpropan-1-ol
Solution
The isomers are those species that contain the same molecular formula. We need to draw the structures of all the compounds given to us in the question and then calculate the number of carbon, hydrogen and oxygen atoms present in the structures. After this, we can derive their molecular formula and thus check whether it is similar to the given in question.
Complete step by step answer:
The alcohol is one that contains OH groups. The isomers are the molecules that have the same molecular formula but different arrangement of atoms in space.
Thus, we have to see the molecules given in options. The option of having both the molecules with C4H10O molecular formula will be the correct answer.
So, let us see the options one by one.
The first option contains the molecules tert-butanol and 2-methylpropan-2-ol. The structures of these molecules are as given below.
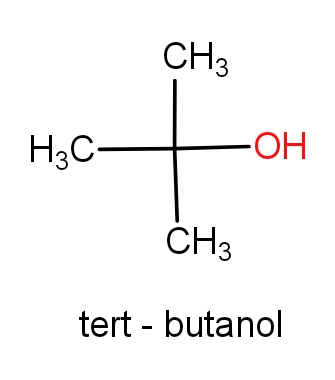
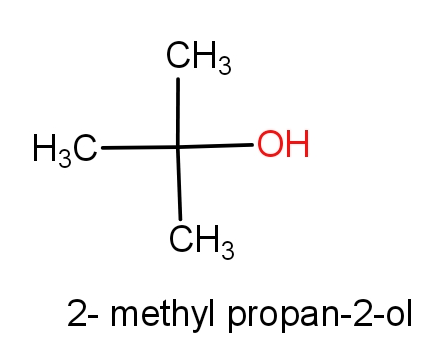
Both the molecules have the same structures but names are different. These both the molecules have C4H10O molecular formula. So, this can be the correct answer.
The second option is tert-butanol and 1,1-dimethyl ethan-1-ol. The structures of these molecules are as given below.
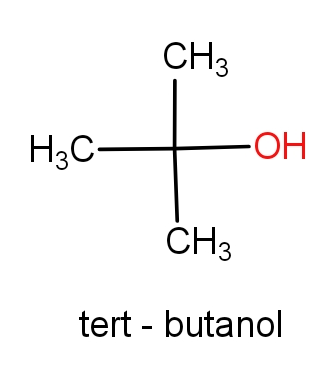
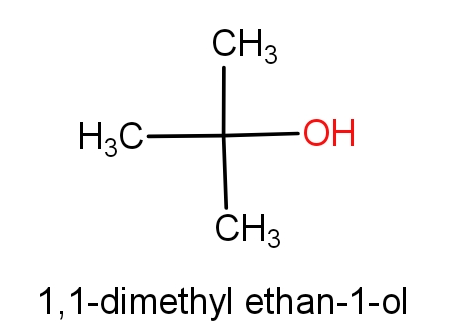
Both the molecules have the same structures but names are different. These both the molecules have C4H10O molecular formula. So, this can be the correct answer.
The third option given contains n-butanol and butan-1-ol. The structures of these molecules are as given below.
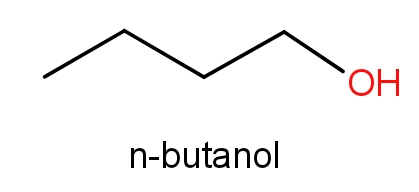
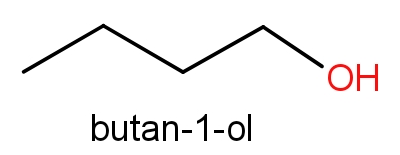
Both these molecules even have the same structures but names are different. These both the molecules have C4H10O molecular formula. So, even this can be the correct answer.
The fourth option has isobutyl alcohol and 2-methylpropan-1-ol.
The structures of these molecules are as given below.
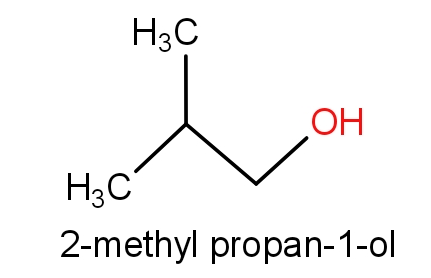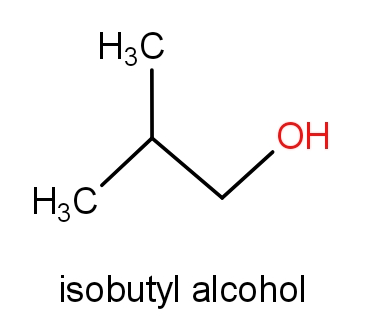
Both the molecules consist of the same structures but names are different. These both the molecules have C4H10O molecular formula. So, this can also be the correct answer.
So, the correct answer is “Option A, B, C and D”.
Note: For drawing the structures from names, one should know about the IUPAC nomenclature of compounds. The word ‘n’ before name is equal to first position only. Further, the word tert means tertiary. Some of the molecules above have IUPAC names while some have common names. So, we should know both the types of names.
