Question
Question: The coordination numbers of Co and Al in \([Co(Cl){{(en)}_{2}}]Cl\) and \({{K}_{3}}[Al{{({{C}_{2}}{{...
The coordination numbers of Co and Al in [Co(Cl)(en)2]Cl and K3[Al(C2O4)3] respectively are:
(en = ethnane-1,2 diamine)
A. 3 and 3
B. 6 and 6
C. 5 and 6
D. 5 and 3
Solution
. The en ligand( ethane 1-2 diamine) is a chelating ligand. So, instead of 1, it occupies 2 bonds with the central metal atom of the coordination compound. Whereas, other compounds which are non-chelating occupy 1 bond with the central metal atom.
Complete step by step answer:
In order to solve the question, let us find out what chelating ligands are. But before that, let us learn about coordination spheres. In coordination chemistry, the primary coordination sphere refers to the array of molecules and ions which are directly attached to the central metal atom of the sphere. The second coordination sphere consists of molecules and ions that are attached in various ways to the primary coordination sphere. Coordination sphere is the basic part of coordination chemistry which deals with the structure of the compounds and their constitution. The coordination sphere can also be related to the atmosphere of the earth, a surrounding that encloses the whole structure.
In a coordination compound, there is a central metal atom. Now there are other atoms attached to this central atom. These attached atoms/groups of atoms are called ligands. Ligands are an active part of the coordination compound and do not get dissolved into ions like salts do. Generally, ligands bind with one bond. But there are special ligands which occupy a space of 2 or more. These special ligands are called chelating ligands. Ethane 1,2 diamine and C2O4 (oxalate) are chelating ligands.
Now, let us look at the structure of the two compounds given in the question:
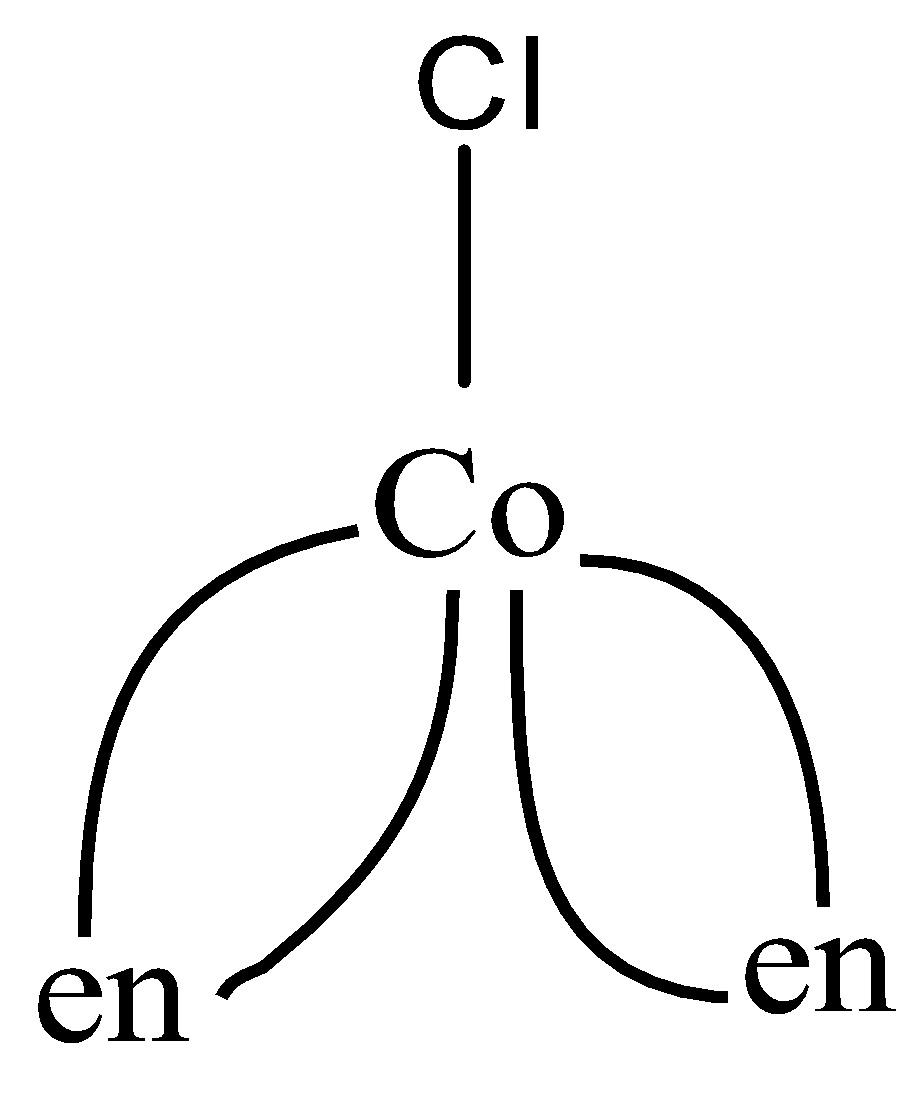
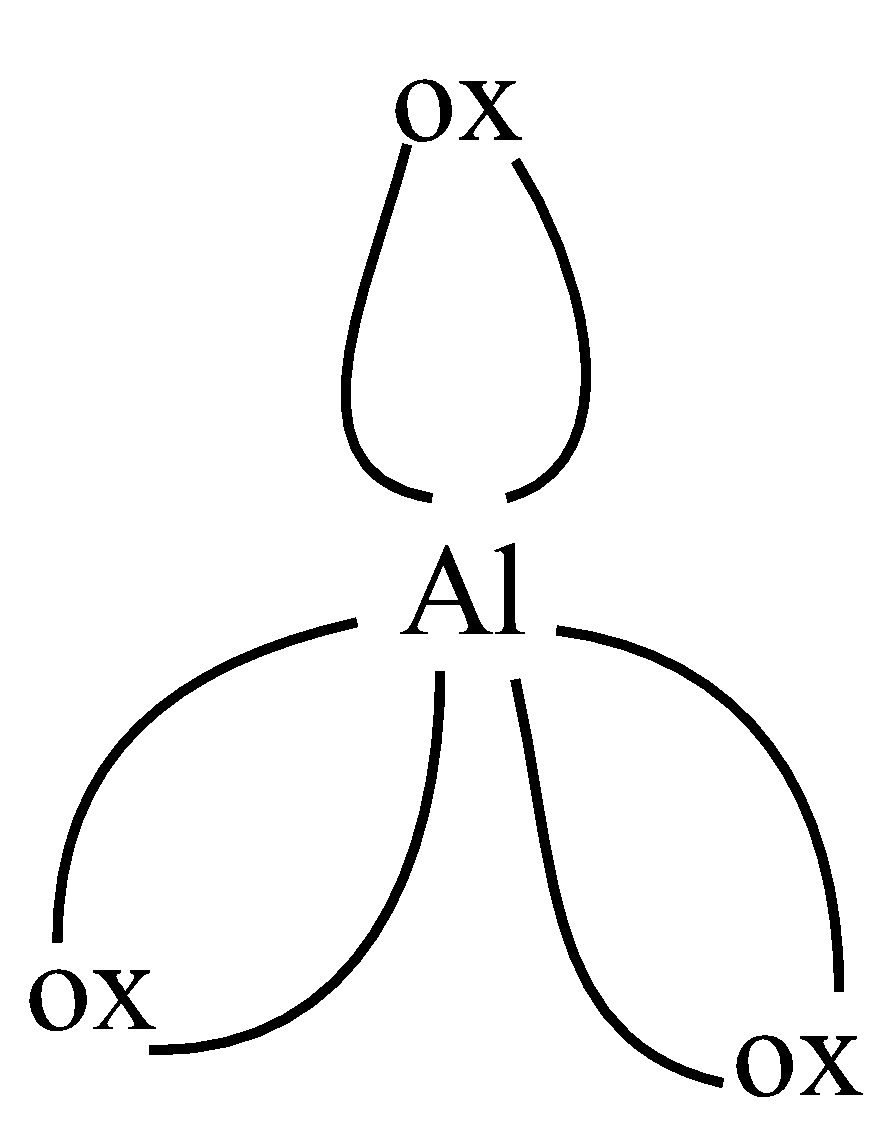
As we can see, in the first figure, coordination number is 5, as 5 bonds are formed and 6 in the second figure, as 6 bonds are formed. So the coordination numbers are 5,6 respectively,
So, the correct answer is “Option C”.
Note: Generally, it is observed that the coordination compounds which have chelating ligands attached to them are more stable than normal ligands. It is due to a phenomenon called the “chelating effect”. More the number of chelating ligands implies more stability of the coordination compound.
