Question
Question: The coordination number of \({\text{Z}}{{\text{n}}^{{\text{ + 2}}}}\) and \({{\text{S}}^{{\text{ - 2...
The coordination number of Zn + 2 and S - 2 ions in the crystal structure if wurtzite are:
1. 4,4
2. 6,6
3. 8,4
4. 8,8
Solution
The coordination number of an atom in a molecule is the number of atoms bonded to an atom. In chemistry and crystallography, the coordination number describes the number of neighbour atoms with respect to a central atom.
Complete step by step answer:
Wurtzite has a hexagonal closest packing structure (hcp), which is characterized by 12 ions in the corners of each unit that create a hexagonal prism.
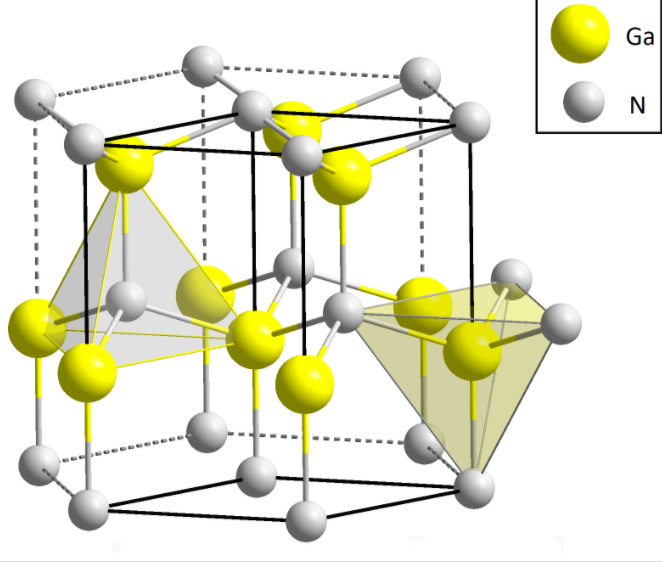
Figure 1: wurtzite
-Zinc blend is a chemical compound and it is available in two different forms : sphalerite and wurtzite.
-These two forms contain 1:1 stoichiometric ratio of Zinc and sulphur atoms which are arranged in the tetrahedral arrangement.
-In the structure the zinc atom is present in the alternate tetrahedral voids and sulphide ions are present in the face -centred of the hexagonal crystal. And these atoms are tetrahedrally coordinated that are staked in an ABABABABAB pattern.
-The coordination number of both zinc and sulphur atoms are 4.
Therefore, the correct answer is (1).
Additional information:
Sphalerite is an important mineral form of zinc sulphide mainly composed of zinc, iron and sulphur atoms. It is mainly occurring in the different kinds of rocks like sedimentary, metamorphic and igneous.ZnS has a unique structure type compared to other molecules. The two forms of this blend are more favourable for thermal conditions.
Note: Wurtzite has hexagonal close-packed crystal structure, in this structure twelve tetrahedral voids are present only six are occupied. cations present in tetrahedral sites. The most important ore of zinc element is the Sphalerite and consists of some variable amount of iron.
