Question
Question: The conversion of acetophenone into benzoic acid can be achieved by its reaction with: A. sodium h...
The conversion of acetophenone into benzoic acid can be achieved by its reaction with:
A. sodium hydroxide followed by acidification
B. iodine and sodium hydroxide, followed by acidification
C. hydroxylamine followed by reaction with H2SO4
D. m-chloroperoxybenzoic acid
Solution
Hint : The above conversion can be achieved by performing iodoform test. The iodoform test is commonly used as a test for the −CH3CO group. The group to which the −CH3CO group is attached can be aryl, alkyl and hydrogen.
Complete step by step solution :
When methyl ketones are treated with the halogen in basic solution, polyhalogenation followed by cleavage of the methyl group occurs.
The products are the carboxylate and trihalomethane, otherwise known as haloform.
The reaction proceeds via successively faster halogenations at the α-position until the 3 H have been replaced.
The halogenations get faster since the halogen stabilizes the enolate negative charge and makes it easier to form.
Then a nucleophilic acyl substitution by hydroxide displaces the anion CX3 as a leaving group that rapidly protonates.
The mechanism for the reaction can be given as:
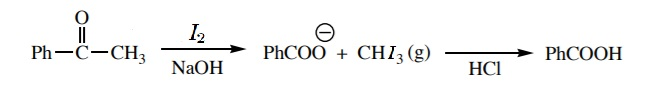
The −CH3CO group is present in acetophenone, when it reacts with iodine in presence of an alkaline medium, the group gets converted to carboxylate ion. On acid hydrolysis, this carboxylate ion gives benzoic acid as the product.
So, the correct option is (b).
Note : This reaction is often performed using iodine and as a chemical test for identifying methyl ketones. Iodoform is yellow and precipitates under the reaction conditions. Iodine in an alkaline medium such as sodium hydroxide or potassium hydroxide act as an oxidizing agent.
