Question
Question: The conversion can be carried out by using the reagent: 
A.H2O,H2SO4
B.O2
C.C6H5COOOH
D.CrO3, H2SO4
Solution
An organic reaction which is used to convert ketone to ester or a cyclic ketone to lactone is called Baeyer- Villiger oxidation. The reagent used in this reaction is peroxy acids. M-CPBA (meta chloro peroxy benzoic acid) or peroxybenzoic acid is used as a reagent in this reaction. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1889.
Complete step by step answer:
Now we will discuss it in details:
-In the given reaction the reactant is a five-membered ring with a ketone (−ROR) group attached to it which is known as Cyclopentanone.
-The product formed in the given reaction is also a cyclic product that has an ester (−COOR) group attached to it and is known as tetra-hydro-2H-pyran-2-one.
-We know that ketone can be converted into ester by using the Baeyer- Villiger oxidation process.
-So the given reaction is an example of Baeyer- Villiger oxidation and the reagent used in this process is peroxide. Therefore, the reagent used here is C6H5COOOH(Peroxybenzoic acid).
-The structure of C6H5COOOH(Peroxybenzoic acid) is
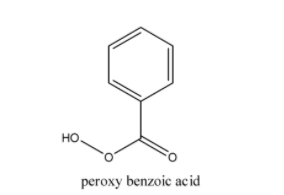
-Hence, the correct option is (C).
Note:
-Ester is characterized by a carbon group attached with two oxygen atoms and another R group. Esters' names are derived from the parent alcohol and the parent acid while the simple esters are often called by their names. All esters can be named by using the IUPAC naming system, based on the name for the acid followed by the suffix “-oate”.
-Ketone is a functional group found in organic chemistry with the structure R−C(=O)−R’ where R and R’ can be a variety of carbon-containing substituents. Ketone contains a carbon-oxygen double bond (carbonyl group). Ketones can be named by using the IUPAC naming system by using the number of carbon atoms followed by a suffix “one”.
