Question
Question: The configuration of the chirality centres in D-threose (shown) are:  are:
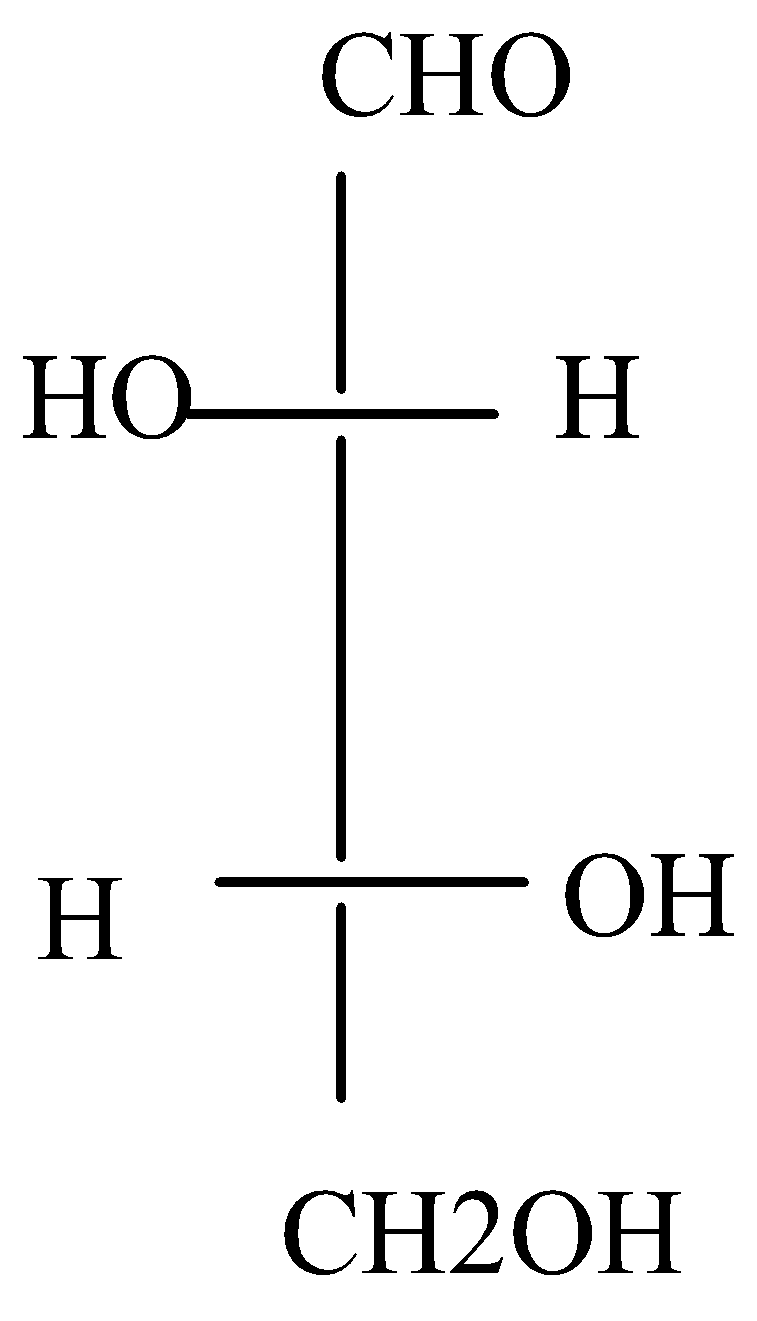
A.2R, 3R
B.2R, 3S
C.2S, 3R
D.2S, 3S
Solution
We know that a counter/anti clockwise direction is an S and a clockwise direction is an R configuration. Here, C is the higher priority and H is the least priority. In D-threose, there is S configuration at Carbon - 2 and there is R configuration at Carbon – 3.
Complete step by step answer:
D-threose is a chiral molecule. D-threose are diastereomers of both D – erythrose and L – erythrose. and they are not the mirror image. It has an enantiomer which is called L-threose which has exactly the same physical properties as D – threose except for one. The two are non-identical but they are mirror images of each other.
In D-threose, there is S configuration at C−2 and there is R configuration at C−3.
A solution of D – threose rotates a beam of plane polarized light to the right, whereas the solution of L – threose rotates the plane to the left.
So, the configuration of the chirality centres in D-threose are 2S, 3R
Therefore, the correct answer is option (C).
Note: The other name of D-threose is Threose, (2S,3R) – 2, 3, 4 dihydroxybutane. Its molecular weight is 120.1 g/mol. Threose is a 4- carbon monosaccharide with molecular formula as C4H8O4
D-threose are diastereomers of both D – erythrose and L – erythrose. Both sugars have two chiral centres. In both of them, the chiral centre farthest from the aldehyde end, carbon 3 has a hydroxyl group on the right side that makes them both D sugars.
For the chiral carbon 2, the hydroxyl group is on the right in erythrose and on the left in threose. That makes them diastereomers, more specifically epimers, since they differ only in the stereochemical configuration at one chiral carbon out of more than one.
