Question
Question: The compounds which uses \(s{{p}^{3}}\)- hybrid orbitals for bond formation are- (A)- \(HCOOH\) ...
The compounds which uses sp3- hybrid orbitals for bond formation are-
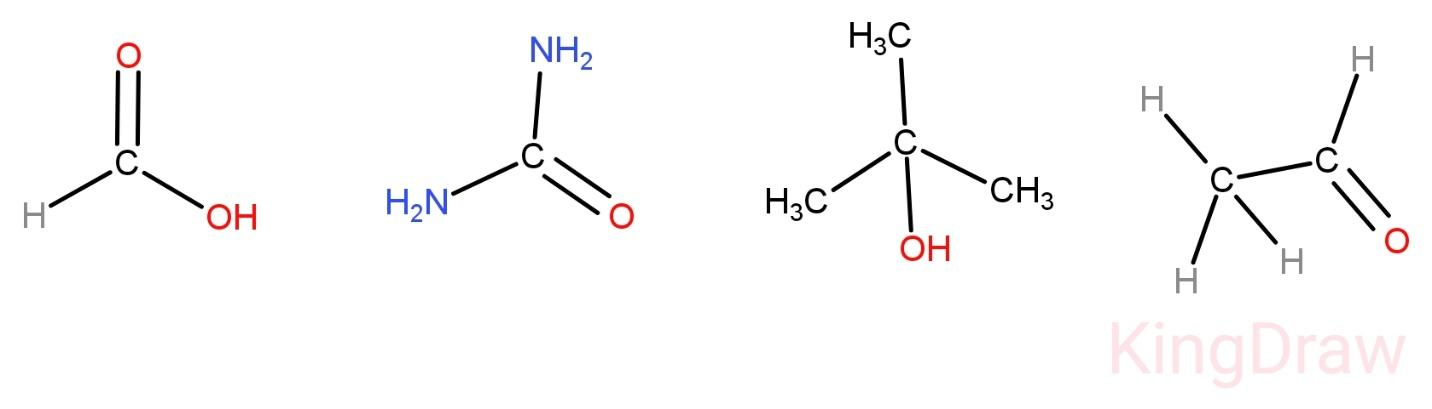
(A)- HCOOH
(B)- (H2N)2CO
(C)- (CH3)3COH
(D)- CH3CHO
Solution
Hint : A sp3- hybridized atom is linked to four other atoms by single bonds (sigma). A sp3- hybrid orbital contains 4 hybrid orbitals. Carbon has tetra valency.
Complete step by step solution :
Let’s look at the given options:
-In HCOOH the carbon atom is linked to 3 atoms only by single bonds. Its hybridization is sp2. So OPTION (A) is incorrect.
- In (H2N)2COthe carbon atom is again linked to 3 atoms only by single bonds. Its hybridization is sp2. Also the 2 N atoms are also linked to 3 atoms only. So this compound does not contain any sp3 hybrid orbital. So OPTION (B) is incorrect.
-In (CH3)3COHall the carbon atoms present are linked to 4 other atoms by single bonds. Its hybridization is sp3. So, OPTION (C) is correct.
-In CH3CHO one carbon atom is linked to 4 other atoms by single bonds. Its hybridization is sp3. So, OPTION (D) is also correct.
So, the correct answer is “Option B and D”.
Note : The geometry of a sp3 hybrid orbital is tetrahedral. The shape of a sp3 hybrid orbital is also the same that is tetrahedral. But if a lone pair is present then the geometry remains the same but the shape changes. Only count single bonds (sigma bonds) while checking hybridization.
