Question
Question: The compound(s) that are soluble in hot aq. NaOH is/are: ...
The compound(s) that are soluble in hot aq. NaOH is/are:
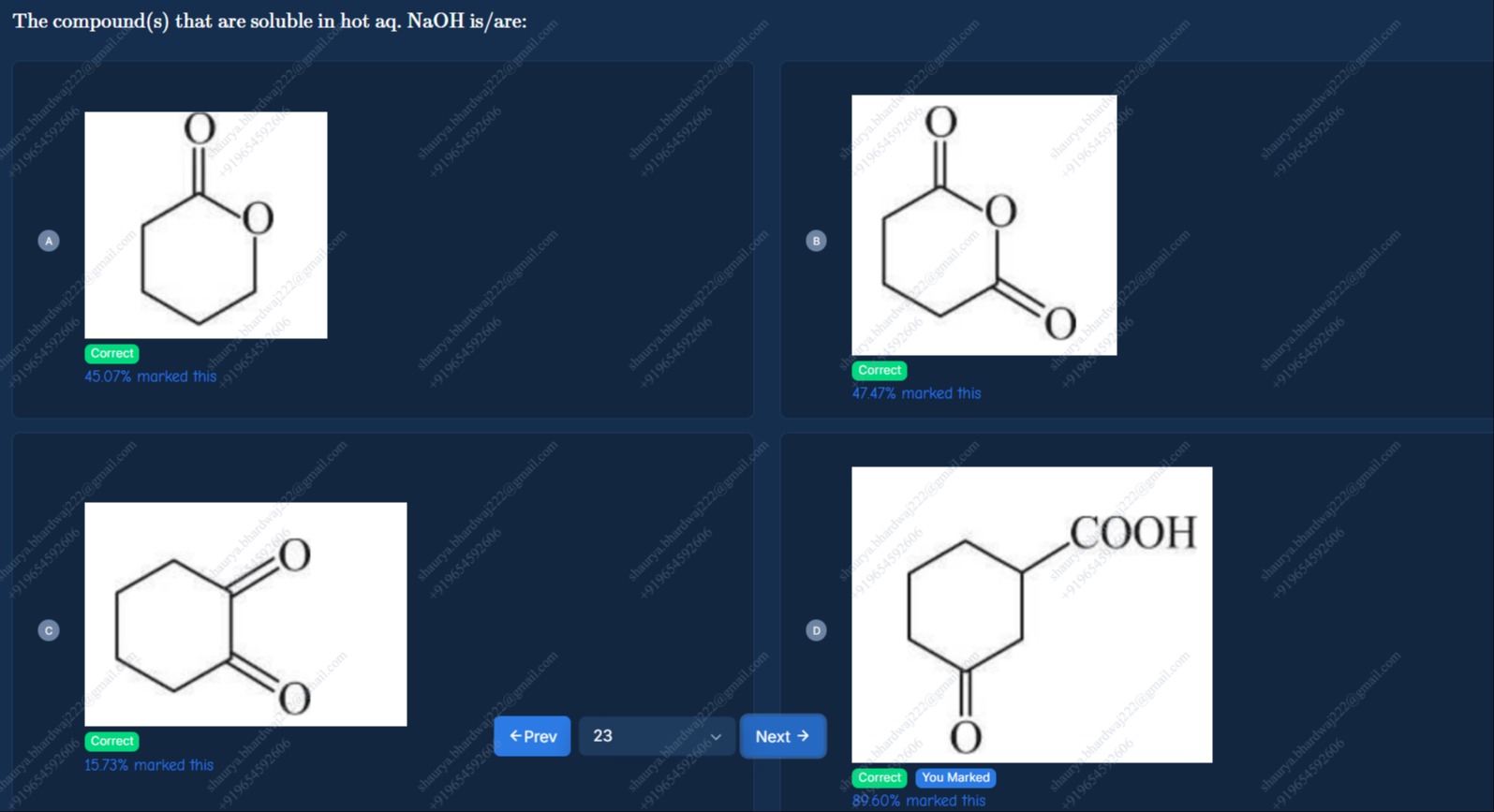
O
//
C
/ \
O CH2
| |
CH2 CH2
| |
CH2 CH2
\ /
CH2
O
//
C
/ \
O O
| |
CH2 CH2
\ /
C=O
O O
// //
C --- C
| |
CH2---CH2
| |
CH2-----CH2
\ /
CH2
COOH
/
CH2-----CH2
| |
CH2 CH2
| |
CH2-------C=O
A, B, C, D
Solution
The solubility of organic compounds in aqueous NaOH depends on the presence of acidic functional groups that can react with NaOH to form water-soluble salts, or functional groups that can undergo hydrolysis in basic conditions to form soluble products. Hot aqueous NaOH provides conditions for both acid-base reactions and hydrolysis reactions.
Let's analyze each compound:
Compound A: This is a cyclic ester (lactone). Esters undergo hydrolysis in basic conditions (saponification) to form a carboxylate salt and an alcohol.
In hot aqueous NaOH, this lactone will be hydrolyzed to form a hydroxycarboxylic acid, which will immediately react with NaOH to form the corresponding sodium salt.
Lactone + NaOH + H2O → Hydroxycarboxylate salt
Hydroxycarboxylate salts are ionic compounds and are generally soluble in water.
Compound B: This compound is a cyclic anhydride. Anhydrides react with water or bases to form carboxylic acids or carboxylate salts.
In hot aqueous NaOH, the anhydride ring will open due to hydrolysis, forming a dicarboxylic acid. This dicarboxylic acid will then react with 2 equivalents of NaOH to form the disodium salt of the dicarboxylic acid.
Cyclic anhydride + 2 NaOH → Disodium salt of dicarboxylic acid
Disodium salts of dicarboxylic acids are ionic compounds and are generally soluble in water.
Compound C: This compound is a 1,2-diketone (1,2-cyclohexanedione). While simple ketones do not react with NaOH to form soluble salts, α-diketones can undergo the benzilic acid rearrangement in the presence of a strong base and heat.
1,2-cyclohexanedione undergoes benzilic acid rearrangement in hot aqueous NaOH to form 1-hydroxycyclohexanecarboxylic acid, which then reacts with NaOH to form the sodium salt of 1-hydroxycyclohexanecarboxylic acid.
1,2-cyclohexanedione + NaOH → Rearranged product (1-hydroxycyclohexanecarboxylic acid)
1-hydroxycyclohexanecarboxylic acid + NaOH → Sodium 1-hydroxycyclohexanecarboxylate + H2O
The sodium salt of 1-hydroxycyclohexanecarboxylic acid is an ionic compound and is soluble in water.
Compound D: This compound contains a carboxylic acid group and a ketone group.
Carboxylic acids are acidic and react with NaOH to form carboxylate salts.
Carboxylic acid + NaOH → Carboxylate salt + H2O
The ketone group does not react with NaOH to form a soluble salt under these conditions. However, the presence of the carboxylic acid group makes the compound acidic, and it will react with NaOH to form the sodium carboxylate salt.
Sodium carboxylate salts are ionic compounds and are generally soluble in water.
Since compounds A, B, C, and D all undergo reactions with hot aqueous NaOH to form water-soluble salts, all of them are soluble in hot aqueous NaOH.
