Question
Question: The compound ‘X’ gives brisk effervescence of \[C{O_2}\] on treatment with \[NaHC{O_3}\] and when tr...
The compound ‘X’ gives brisk effervescence of CO2 on treatment with NaHCO3 and when treated with LiAlH4 gives ethyl alcohol. Therefore ‘X’ may be..
(A) CH3COCH3
(B) CH3CH2CHO
(C) CH3CHO
(D) CH3COOH
Solution
Lithium aluminum hydride reacts with carbonyl carbon to give hydroxyl group in product. Sodium bicarbonate is a base and it reacts with acids to give salts. Both aldehydes and carboxylic acid groups will get reduced by lithium aluminum hydride.
Complete step by step answer:
It is proved from experiments that organic acids produce carbon dioxide gas when they react with sodium bicarbonate solution. So, salt of acid and carbon dioxide gas are produced as products. The release of carbon dioxide gas gives brisk effervescence. Let’s see which of the given compounds will give brisk effervescence when treated with sodium bicarbonate.
- CH3COCH3 contains only ketone functional group, CH3CH2CHO and CH3CHO contains aldehyde functional group. So, these three compounds will not react with sodium bicarbonate.
- CH3COOH has a carboxylic acid group and is an organic acid, so it will react with sodium bicarbonate to produce brisk effervescence of carbon dioxide gas. The reaction is given as below.
Acetic acidCH3COOH+NaHCO3→Sodium AcetateCH3COONa+CO2
Now, Lithium aluminum hydride is a reducing agent and will reduce any carbonyl carbon to give hydroxyl group on it.
Let’s see how the given compounds will react with Lithium aluminum hydride.
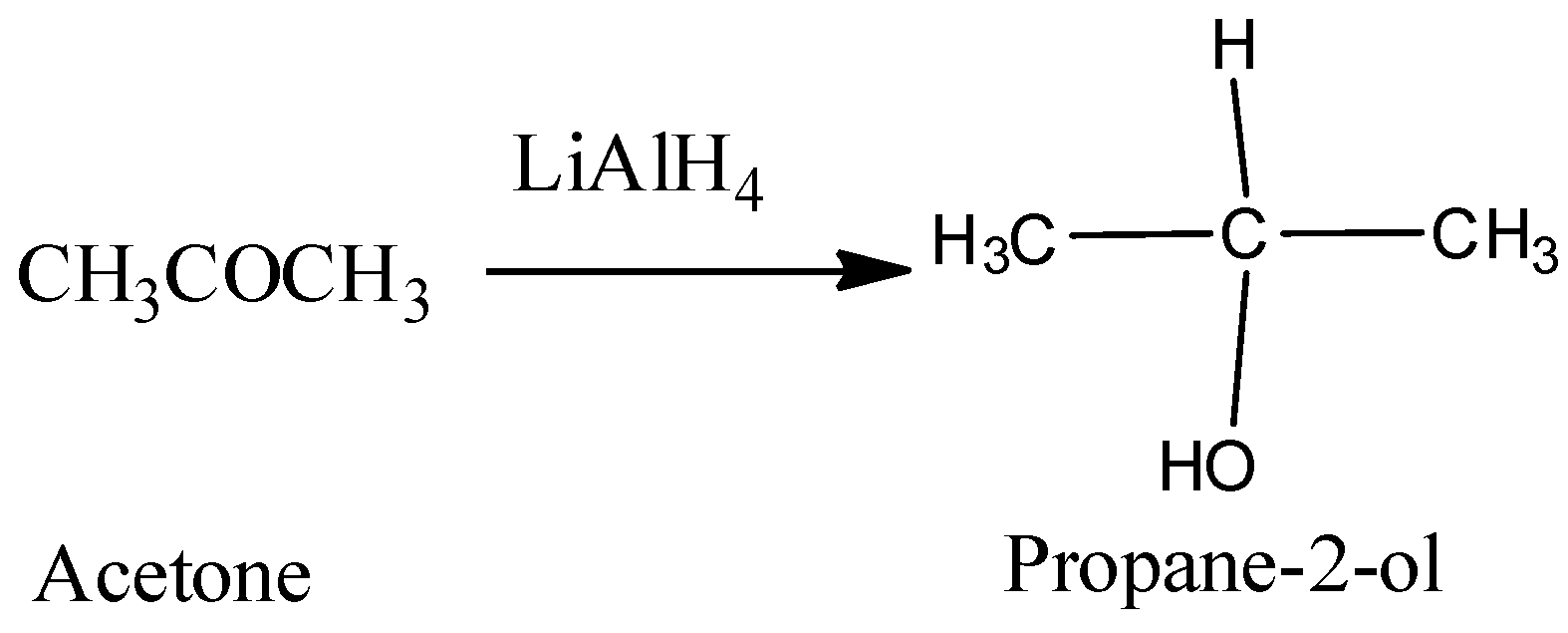
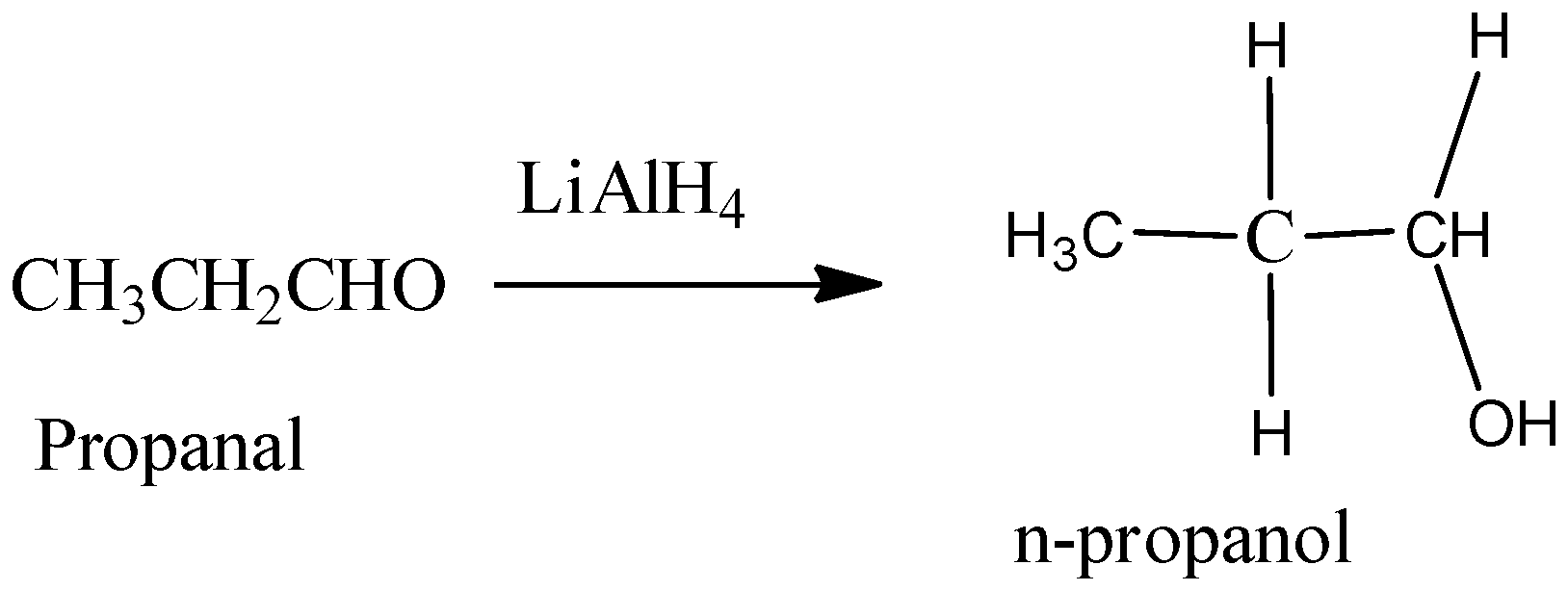
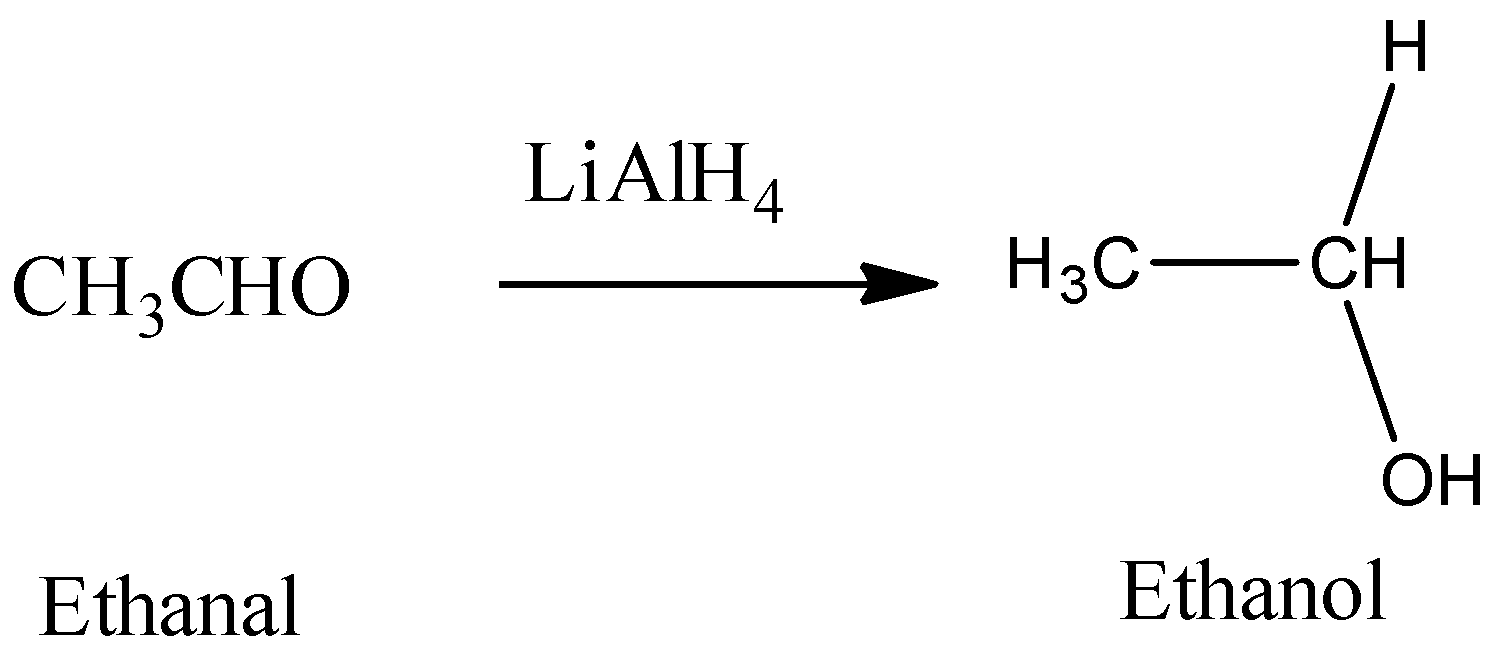
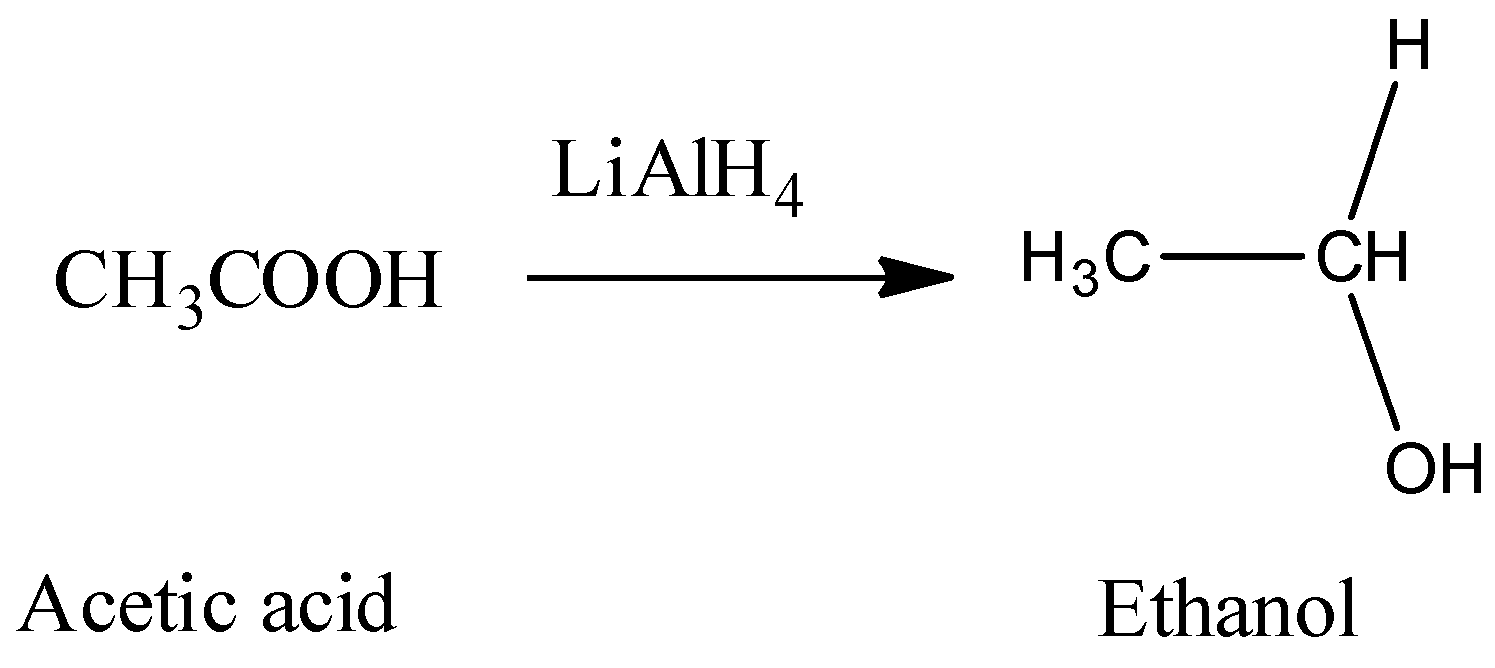
Hence, from the above reaction, we can see that acetone forms 3-propanol, Propanaldehyle forms 1-propanol and Acetic acid and acetaldehyde will react with lithium hydride to produce ethanol.
- So, only the compound that gives carbon dioxide with sodium bicarbonate and gives ethanol with lithium aluminum hydride is Acetic acid.
So, correct answer is (D) CH3COOH.
Note:
Remember that hydrogen atoms of aldehydes are not acidic and they cannot react with sodium bicarbonate to give carbon dioxide gas. Remember that lithium aluminum hydride converts –CHO and –COOH groups to −CH2OH groups.
