Question
Question: The compound, whose stereo chemical formula is written below, exhibits x geometrical isomers and y o...
The compound, whose stereo chemical formula is written below, exhibits x geometrical isomers and y optical isomers.
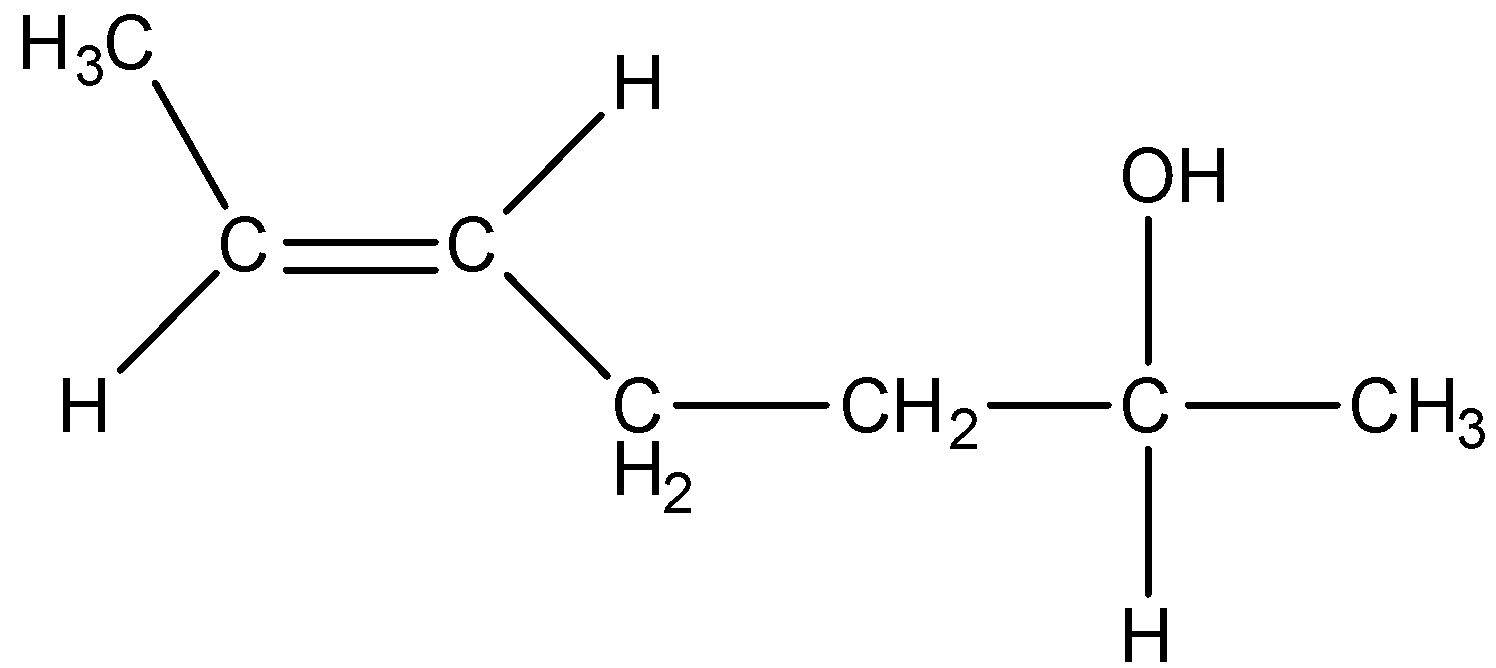
The value of x and y are:
A.4 and 4
B. 2 and 2
C. 2 and 4
D. 4 and 2
Solution
Hint Optical activity of an organic compound is dependent upon the presence of chiral carbon that is present in it. If all the four valencies of carbon are satisfied by 4 different groups the carbon is chiral carbon or asymmetric carbon.
Complete answer:
If all the four valencies of carbon are satisfied by 4 different groups the carbon is chiral carbon or asymmetric carbon. Presence of chiral carbon never ensures the molecule is optically active.
Compounds with one chiral carbon are always chiral. While compounds containing more than one chiral carbon may or may not be chiral.
Whereas achiral carbon is a carbon which is optically inactive and in which there is at least 2 same substituents are present.
Any molecule is optically active if
(a) it is non-superimposable on its mirror image.
(b) does not contain any element of symmetry
(1) Plane of symmetry (o): Any imaginary plane which divides the full molecule into two halves which are mirror images of each other.
(ii) Centre of inversion : Any imaginary point, through which when inversion will be carried out it will repeat equivalent configuration.
(iii) Alternating axis of symmetry (S.): A molecule posses an alternating axis of symmetry, if when rotated through an angle of n360 about the axis and then followed by reflection in a plane perpendicular to the axis, molecule comes to its equivalent form.
Molecules which possess non-superimposable mirror images of one other are called enantiomers.
(0) Enantiomers have the same physical properties except their optical rotation which is equal in magnitude but in the opposite direction.
(ii) Enantiomers have the same chemical behaviour towards the molecules which are optically inactive, but they have different chemical behaviour towards the molecules which are optically active.
Now, let us come to our question. Speaking of geometrical isomers, there can be only 2 geometrical isomers possible as compound contains only one double bond and can posess both cis and trans configuration. Now, for finding optical isomers, we need to find the chiral carbons. We can observe that in the later part of the chain, the 5 numbered carbon is optically active as it has four different groups present. So, we obtain the number of optical isomers as 2n=21=2 optical isomers.
Hence, we obtain the correct answer as option B.
Note: 1:1 molar mixture of laevo and dextro forms of the same enantiomeric pair is not optically active as the optical rotation due to dextro form is negotiated by the optical rotation due to laevo form and this mixture is called racemic mixture.. It is the case of external compensation.
