Question
Question: The compound which has one isopropyl group is: (A) 2, 2, 3, 3-tetramethylpentane (B) 2, 2-dimeth...
The compound which has one isopropyl group is:
(A) 2, 2, 3, 3-tetramethylpentane
(B) 2, 2-dimethylpentane
(C) 2, 2, 3-trimethylpentane
(D) 2-methylpentane
Solution
Hint: To answer this question, we should first draw the structure of each of the organic compounds that are present in the option. We should understand that Isopropyl group is nothing but propane with hydrogen from middle C-atom removed that is CH(CH3)2.
Complete step by step solution:
So, first we should discuss the isopropyl group. We should know that isopropyl group is a portion of a molecular structure propyl which is a three-carbon alkyl substituent with chemical formula −CH2CH2CH3. And it is equivalent to propane minus one hydrogen atom from the middle carbon.
Now, we will draw the structure of each option and we will find which compound has isopropyl group.
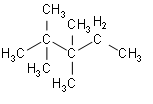
- Structure of the first option 2, 2, 3, 3-tetramethylpentane is:
The above represented structure doesn’t have any isopropyl group.
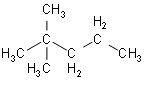
- Now, we will look at the second option of 2, 2-dimethylpentane:
The above represented structure doesn’t have any isopropyl group.
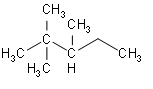
- Now, we will look at the third option of 2, 2, 3-trimethylpentane.
This structure doesn’t also have any isopropyl group.
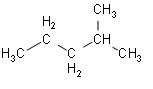
- Now, we look at the fourth option of 2-methylpentane.
This structure contains the isopropyl group. So, from this structure we can say that the fourth option is the correct option.
Note:
We should note that 2-Methylpentane is a branched-chain alkane. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. We should note that the formula of 2-methylpentane=C6H14. By looking at the formula, it looks like it has no degrees of unsaturation. The formula falls under the classCnH2n+2. We should note that 2-methylpentane is thus a saturated molecule, with neither double bond, nor ring junctions.