Question
Question: The compound which can be used to prepare iodoform is: A.Methyl iodide B.Acetone C.Propionalde...
The compound which can be used to prepare iodoform is:
A.Methyl iodide
B.Acetone
C.Propionaldehyde
D.Acetic acid
Solution
Iodoform or triiodomethane is an organic compound with the formula CHI3 and it is analogous to chloroform.
Complete step by step answer:
Iodoform can be prepared by the iodoform reaction. The iodoform reaction refers to the chemical reaction in which a methyl ketone is subjected to oxidation by allowing it to react with aqueous sodium hydroxide (NaOH) and iodine (I2) to form a carboxylate. The iodoform reaction also produces a yellow solid which precipitates from the mixture. This yellow solid produced from the iodoform reaction is called iodoform. Since acetone is a methyl ketone, it can be used to prepare iodoform.
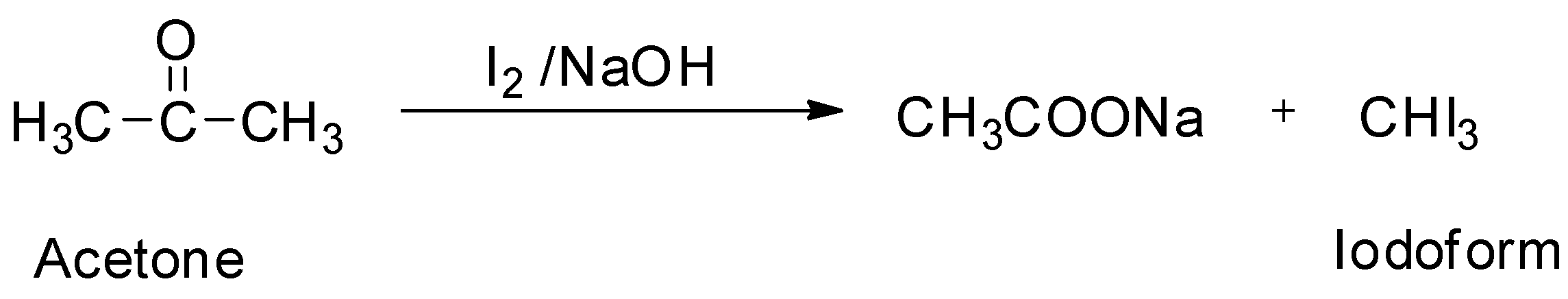
So ,option B is correct.
The other three options do not define a methyl ketone and so they are incorrect.
Additional information: (1) Iodoform can also be prepared from some other organic compounds like ethanol (C2H5OH), acetaldehyde (CH3CHO) and secondary alcohols (CH3CHROHwhere R represents an alkyl or aryl group) in place of a methyl ketone. Iodoform is synthesized by treating any of these four compounds with iodine (I2) and sodium hydroxide (NaOH) in the haloform reaction.
(2)Iodoform is often used as a disinfectant, mainly in hospitals. It was also earlier used in medicine for treating wounds as a healing and antiseptic dressing.
(3)A chemical test called iodoform test is used to check the presence of a methyl ketone moiety in an unknown substance. The presence of methyl ketone moiety means the presence of carbonyl compounds having the structure CH3COR or alcohols having the structure CH3CHROH where R is an alkyl or aryl group. The pale yellow precipitate of iodoform produced in this test confirms the presence of methyl ketone. The only aldehyde which gives a positive iodoform test is acetaldehyde since it contains a CH3CO - group.
Note:
Acetone is a methyl ketone since it has the structure CH3COR where R is a methyl group.
