Question
Question: The compound which as one isopropyl group is: (A) 2, 2, 3, 3- tetramethyl pentane (B) 2,2- dimet...
The compound which as one isopropyl group is:
(A) 2, 2, 3, 3- tetramethyl pentane
(B) 2,2- dimethyl pentane
(C) 2, 2, 3 –trimethylpentane
(D) 2-methyl pentane
Solution
The nomenclature is a very important part of organic chemistry. This nomenclature is not only for compounds but also for carbon atoms that make this compound. For nomenclature of carbon atoms, use the terms primary, secondary, and tertiary to refer to the substitution level that carbon has in a molecule. based on the classification of carbon atoms useful to determine the stability and predict the products in organic reaction mechanisms.
Complete step by step solution:
Classifications of carbon atoms based on nomenclature are primary, secondary, tertiary, and quaternary. To describe how many other carbons are attached to given carbon. This classification applied to only saturated carbons and its substitution compounds like alcohols, amines, etc.
The terminology of carbon-containing functional groups:
Primary carbons: a hydrogen atom on a carbon atom attached to one other carbon atom
Secondary carbons: hydrogen on a carbon attached to only two other carbon atoms
Tertiary carbons: hydrogen on a carbon attached to three other carbon atoms
Quaternary carbons: carbon attached to four other carbon atoms.
If any carbon atom is a tertiary carbon with this structure will be
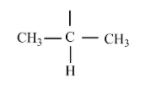
Let us check the IUPAC names of the compounds given,
(A) 2, 2, 3, 3- tetramethyl pentane – there is no isopropyl carbon atom in the parent chain.
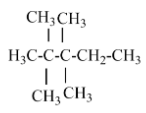
(B) 2,2- dimethyl pentane – there is no isopropyl group in this structure
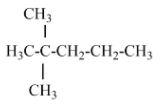
(C) 2, 2, 3 –trimethyl pentane - there is no isopropyl group in this structure
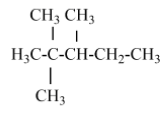
(D) 2-methyl pentane- contains one isopropyl group and Option D is the correct answer.
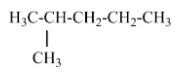
Hence, the answer is option (D).
Note: For carbocations, if carbons with more carbons attached which is tertiary carbocation tend to be less electron deficient due to hyperconjugation from nearby C-H bonds. So, tertiary carbon cations are more stable compared to secondary, primary, and methyl cations respectively.
