Question
Chemistry Question on Polarity of bonds
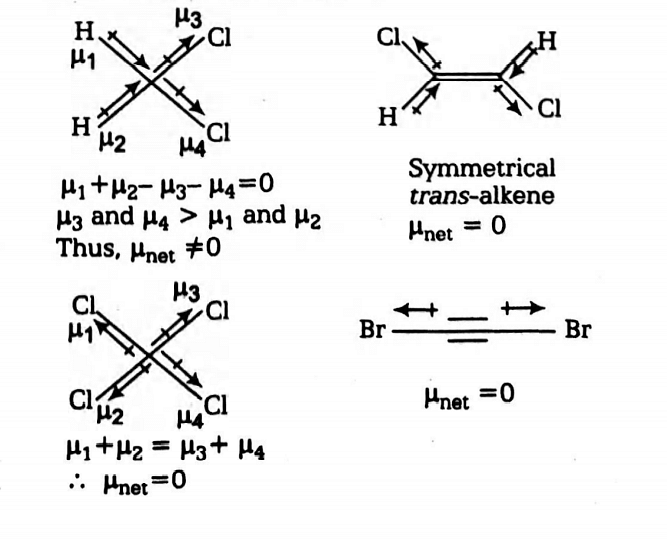
The compound that will have a permanent dipole moment among the following is
A
I
B
II
C
III
D
IV
Answer
I
Explanation
Solution
Symmetrical molecules and symmetrical trans-alkene have a net dipole moment zero.
CH2Cl2 is not a symmetrical molecule, thus it will have a permanent dipole.