Question
Question: The compound that is most difficult to protonate is: A.
B.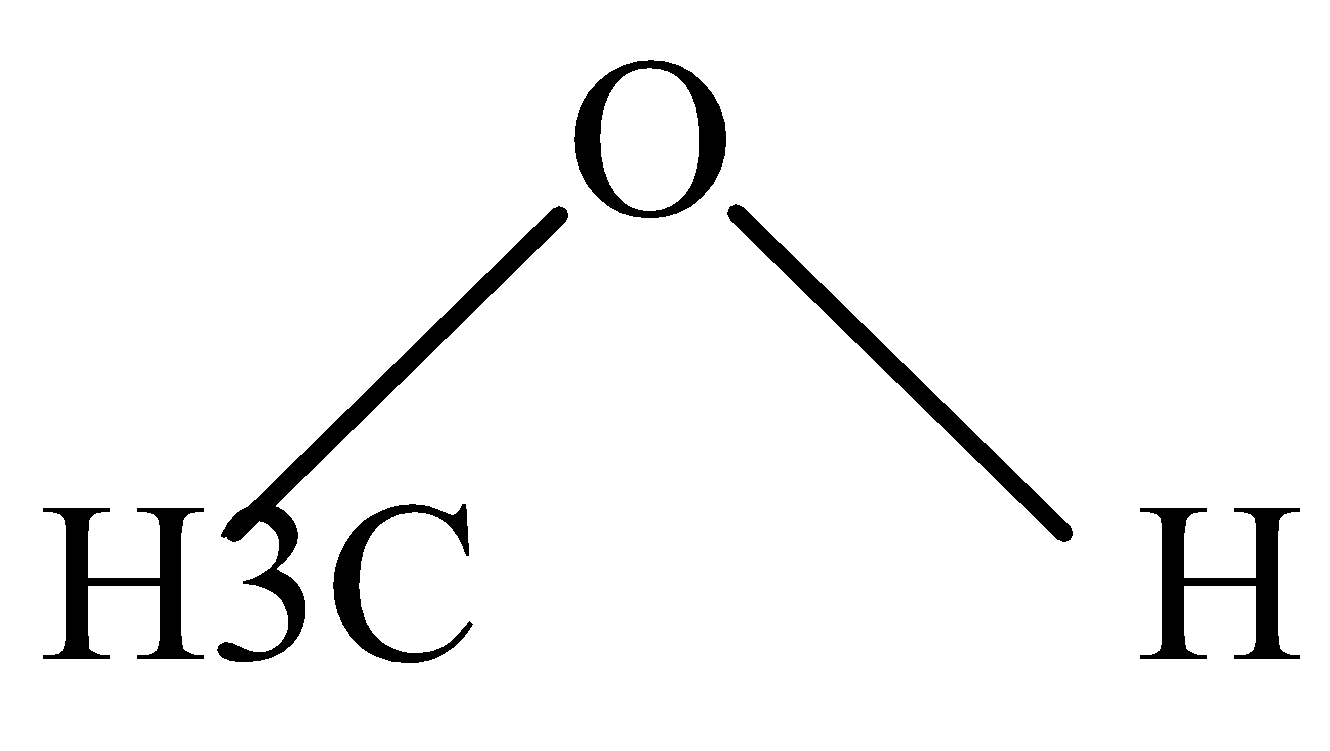
C.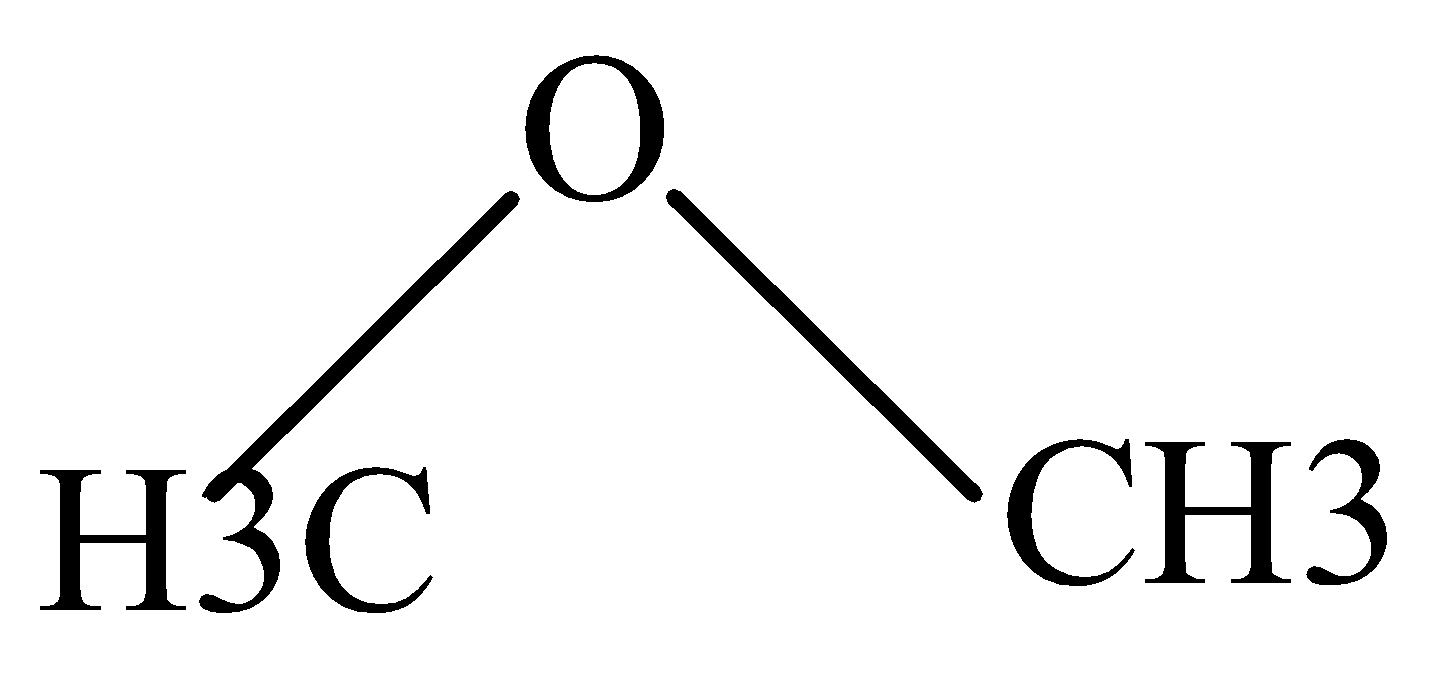
D.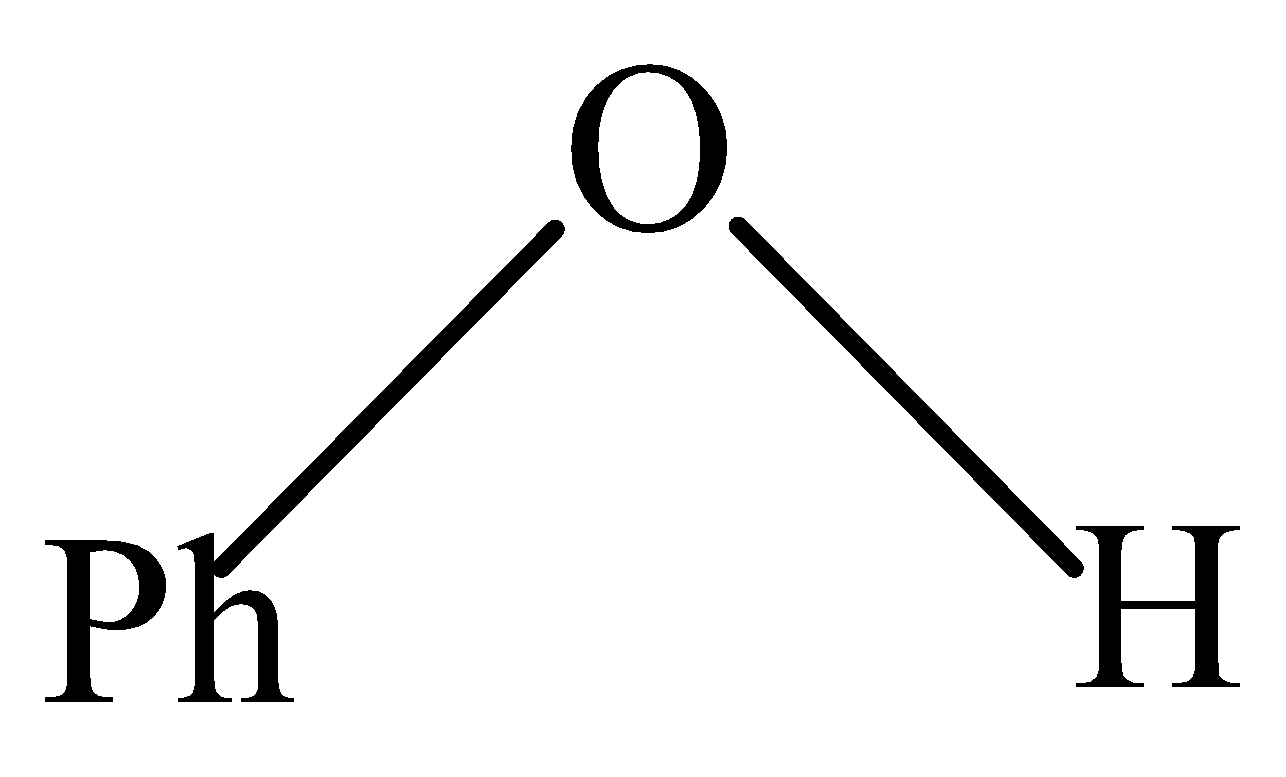
Solution
Protonation takes place on an Oxygen atom due to a lone pair of electrons. In phenols, the lone pair of oxygen is in resonance with the benzene ring.
Complete step by step answer:
In H2O , protonation takes place easily because of the lone pair of electrons that are available for protons to attack easily.
In CH3OH and CH2OCH3 , the Protonation takes place easily because of the +I effect of methyl groups which increases electron density on oxygen and makes proton to attack easily.
In Phenol, the O−H group is directly attached to the benzene ring. The lone pair of electrons present on the Oxygen of O−H is shared with a benzene ring through resonance thereby making partial positive (+ve) charge on Oxygen. Hence, attacking an incoming proton is not that easily, thus making it most difficult.
So, the compound that is most difficult to protonate is Phenol.
Therefore, the correct answer is option (D).
Note: It should be noted that protonation in alcohols (R−O−R) are easy usually except in phenol. Protonation is the addition of a proton H+ to an atom, molecule or ion forming the conjugate acid. It occurs in many catalytic reactions and it is usually a reversible chemical reaction. Protons are rapid because of the high mobility of protons in the most solvents. When a species is either protonated or deprotonated, its mass and charge change, and also its chemical properties are changed. For example, protonation may change the optical properties, hydrophobicity, or reactivity of a substance. Protonation is usually a reversible chemical reaction.
