Question
Question: The compound \(\text{ CuCl }\) has a zinc blende structure and the edge length of its unit cell is 5...
The compound CuCl has a zinc blende structure and the edge length of its unit cell is 500 pm, its density (in g cm3− ) is :
(Given that the atomic weight of Cu is 63.5 amu and of Cl is 35.5 amu.)
A) 5.24
B) 1.14
C) 2.99
D) None of the above
Solution
The density of compound CuCl can be calculated by the formula of density in consideration with the cubic lattice. The relation is given as follows:
Density = unit cell volume×Avogadro !!′!! s No.Mass ×Z
Where , Z is the number of atoms in the crustal structure.
Here, CuCl has a zinc blende structure. Thus ,the number of atoms per unit in the CuCl structure would be equal to the number of units of atoms in the zinc blend structure.
Complete Solution :
We are provided the following data in the problem.
The molar mass of copper chloride CuCl is equal to the sum of the molar mass of the copper and chloride. Molar mass of CuCl = mass of Cu + mass of Cl = 63.5 + 35.5 = 99.0 amu
- The edge length ‘a’ of the copper chloride compound is equal to ,
a = 500 pm = 500 × 10−12 m
Here ,we wan to determine the density in terms of cm thus ,convert the meters into the cm as ,
1m = 100 cm = 102cm
Therefore edge length becomes:
a = 500 × 10−12 m = 500 × 10−12×102 cm = 500 ×10−10cm
- We have to find the Density of the crystal structure.
The density of the crystal structure is related to the edge length .The relation is given as follows:
d = a3 !!×!! NA Z×M
Where , d is the density of the crystal structure
Z is the number of atoms per unit structure
M is the molar mass of the compound
‘a’ is the edge length of the crystal structure and
NA is the avogadro's number .
- We know that the number of units per cell is 4, it means that it has a face-centered cubic (fcc) structure.
Lets substitute the values in the formula of density, then it can be written as:
d = a3 !!×!! NA Z×M d = (500×10−10)3×6.02×10234×99 d = 75.287396= 5.24 g cm−3
Therefore , Density of copper chloride is 5.24 g cm−3
So, the correct answer is “Option A”.
Note: In copper chloride CuCl structure, the copper is situated at the corners and the center of each face of a cubic structure. The chlorine atoms are placed in between the copper atoms. In copper chloride, the copper and chloride are arranged in ABAB arrangement.It resembles the zinc blend structure.
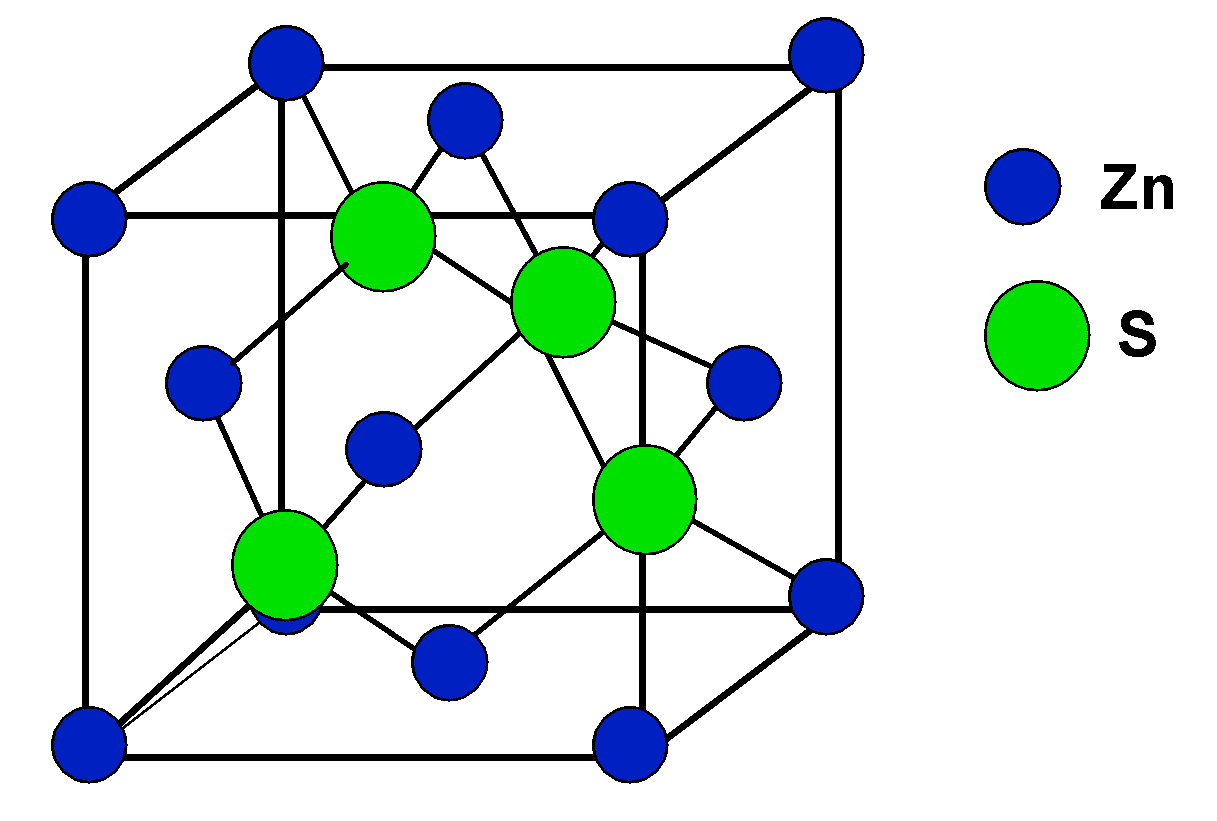
There is no need to get confused by the zinc blende structure, it is just a trick. The structure of the zinc blend ZnS is given as follows,
It relates to the fcc structure of the unit cell, and that will help in identifying the value of Z, i.e. the number of units per cell, which is 4.
