Question
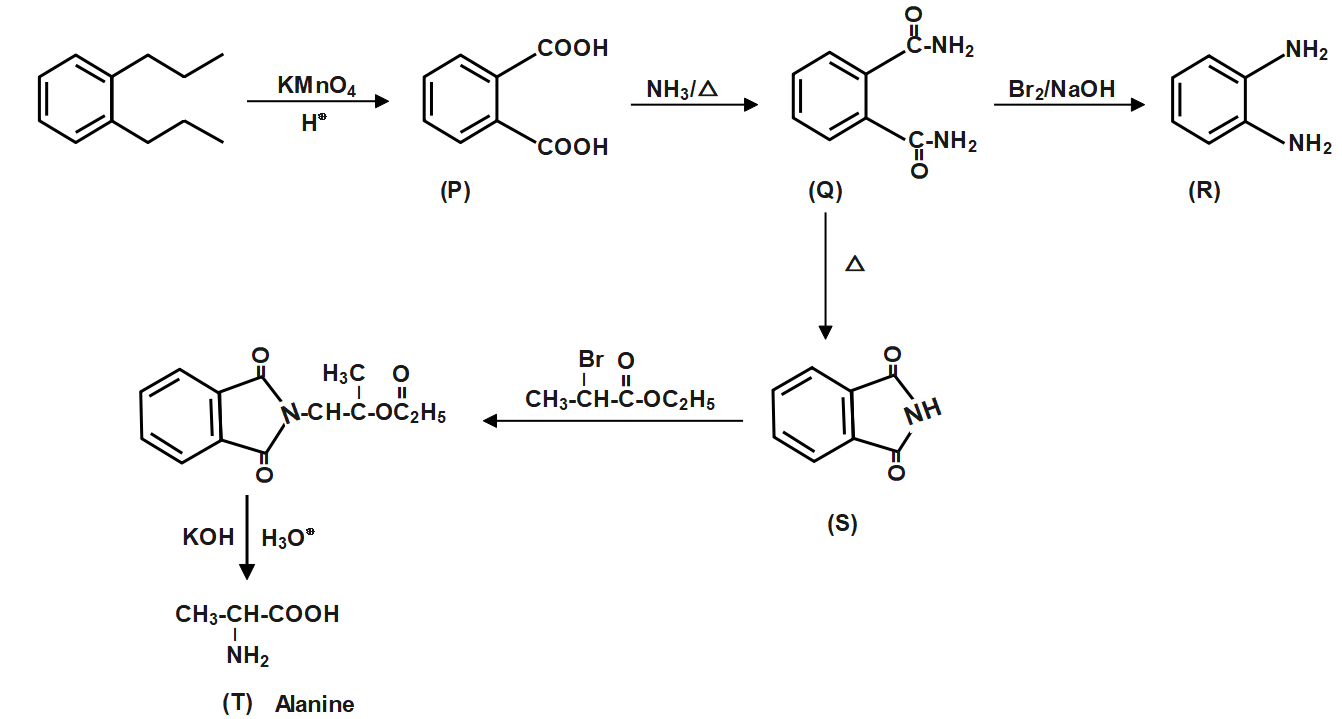
Question: The compound T is: 
(A) Glycine.
(B) Alanine.
(C) Valine.
(D) Serine.
Solution
The resonance structures of Alanine are drawn by first displacing the lone pair of electrons on the nitrogen to the bond between C and N. This results in formation of a double bond between C and N with N getting a positive charge due to the donation of electrons.
Complete answer:
Alanine has the chemical formula CH3−CH(NH2)−COOH. It is an organic compound that consists of a phenyl group attached to an amine group. Alanine is considered to be the simplest aromatic amine. Alanine has the odour of a rotten.
The chemical reaction is given by;
The valency of C is 4. This means that C can form 4 bonds. As C−1 already had 4 bonds, the electrons on C=C pi bond move to the adjacent C−2 atom and the C−2 atom gets a negative charge. This is how the second structure is formed.
Similarly, the negative on the C−2 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to the C−4 atom and it gets a negative charge. This is how the third structure is formed.
Next, the negative on the C−4 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to the C−6 atom and it gets a negative charge. This is how the T structure is formed.
Therefore, Correct answer is option B i.e., Alanine.
Note:
Alanine has a wide range of uses.
-It is used in rubber accelerators and antioxidants.
-Alanine is also an ingredient in dyes.
-It is used in pharmaceuticals and petroleum refining
