Question
Question: The compound(s) that exhibit(s) geometrical isomerism is (are): A. \([Pt(en)C{{l}_{2}}]\) B. \(...
The compound(s) that exhibit(s) geometrical isomerism is (are):
A. [Pt(en)Cl2]
B. [Pt(en)2]Cl2
C. [Pt(en)2Cl2]Cl2
D. [Pt(NH3)2]Cl2
Solution
Geometrical isomerism is going to exhibit a compound which has different ligands around the central metal atom. If all the ligands are the same around the central metal atom then the molecule should not show any geometrical isomerism.
Complete answer:
- Coming to the given question we have to find which molecules in the options show geometrical isomerism.
- Coming to option A, [Pt(en)Cl2] , it does not show any geometrical isomerism due to the less number of ligands attached to the central metal atom. So, option A is wrong.
- Coming to option B, [Pt(en)2]Cl2 , it is also does not show any geometrical isomerism because the chlorine atoms are present in the outer sphere complex, they should present in inner sphere complex to show geometrical isomerism. So, option B is also wrong.
- Coming to option C, [Pt(en)2Cl2]Cl2 . The possible structures by this compound are as follows.
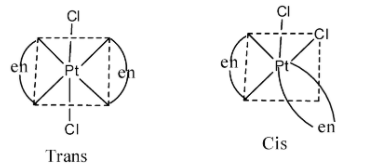
- So, option C shows geometrical isomerism.
-Coming to option D, [Pt(NH3)2]Cl2 . The possible structures by this compound are as follows.
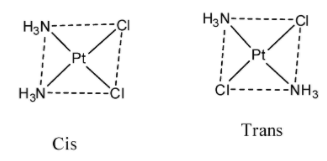
- So, the compound D also shows geometrical isomerism.
Therefore options C and D shows geometrical isomerism among the given options.
Note:
In case of coordination complexes the cis and trans forms can be identified by the presence of ligands around the central metal atom. If same ligands are adjacent in the structure of the complex then the structure is called cis and same ligands are opposite in the structure of the complex then the structure is called trans.
