Question
Question: The compound on reaction with \( NaI{O_4} \) in the presence of \( \;KMn{O_4} \) gives 
A) CH3COCH3
B) CH3COCH3+CH3COOH
C) CH3COCH3+CH3CHO
D) CH3CHO+CO2
Solution
Hint : Here the reactant is alkene which reacts with Lemieux reagents. KMnO4 is a strong oxidizing agent which oxidizes alkene to cis-diol and NaIO4 cleaves the bond between two carbon atoms of the obtained product.
Complete Step By Step Answer:
The given molecular formula is- CH3−C(CH3)=CH−CH3
It is also called is isopentane and its IUPAC name is 3−methylbut−2−ene
This compound reacts with given reagents- sodium per-iodate and potassium permanganate. . The aqueous solution of both the reagent is known as Lemieux reagent
We have to find the product.
Here, KMnO4 is oxidizing agent so it oxidizes the alkene and cis-diol product is obtained. The reaction is given as-
CH3−C(CH3)=CH−CH3KMnO4CH3−C(CH3)(OH)−CH(OH)−CH3
This obtained product is then cleaved by sodium periodate into aldehyde and ketone. The reaction is given as-
CH3−C(CH3)(OH)−CH(OH)−CH3NaIO4−H2OCH3COCH3+CH3CHO
Now, since aldehyde is more reactive than ketone it is further oxidized by KMnO4 to carboxylic acid. The reaction is given as-
CH3CHOKMnO4CH3COOH
The whole reaction can be summarized as-
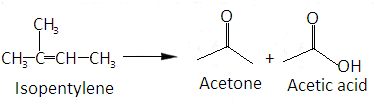
Hence correct answer is option B.
Note :
Students may go wrong if they think that the reaction stops when aldehyde is formed and choose the option C which is incorrect.
We know that KMnO4 is a strong oxidizing agent which can oxidize the reagent in any medium whether it is acidic, basic or neutral so it further oxidizes aldehyde to carboxylic acid. KMnO4 Has following properties-
It is odourless and purple in colour.
It’s aqueous solution, a sweet taste.
It is soluble in water, pyridine, methanol and other organic solvents.
It has a strong oxidizing property so it is used as an oxidant in many reactions.
