Question
Question: The compound \[M{X_4}\] is tetrahedral. The number of \[\angle XMX\] angles in the compound is: A....
The compound MX4 is tetrahedral. The number of ∠XMX angles in the compound is:
A. 3
B. 4
C. 5
D. 6
Solution
The number of bonds gives the information regarding the bond angles in the compound. For this label all the bonded atoms and check for the number of bond pairs and lone pairs.
Complete step by step answer: The compound MX4 is tetrahedral. That means it resembles methane CH4 which also has four hydrogen atoms bonded to the central carbon atom.
In order to determine the number of ∠XMX bond angles the four bonded atoms X are labeled as A, B, C and D. As the compound is tetrahedral all the bond angles in the compound are at an angle of109∘28′.
Let us draw the labeled diagram of the compound MX4.
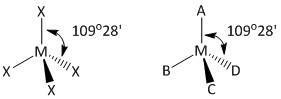
In the tetrahedral model the top X atom is labeled as A, the bottom three are labeled as B, C and D. The atom C is above the plane and the atom D is inside the plane.
The bond angle indicates that the ∠AMD angle is 109∘28′ which is the same as that for ∠XMX angle. Thus the bond angles in MX4 are written as
∠AMB
∠AMC
∠AMD
∠BMC
∠BMD
∠CMD
Hence the number of ∠XMX bond angles in MX4 is six
So, the correct answer is “Option A”.
Note: The bond angle of tetrahedral compound is with the help of VSEPR theory. VSEPR stands for Valence Shell Electron Pair Repulsion theory. It assumes the number of valence electrons around the central atom plus the number of bonded atoms. In tetrahedral geometry the bond pair- bond pair repulsion leads to the bond angle of 109∘28′. If a lone pair is present in spite of a bonded atom the bond angle changes along with the geometry due to greater repulsion between lone pair and bond pair.
