Question
Question: The compound formed as a result of oxidation of ethylbenzene by \({\text{KMn}}{{\text{O}}_{\text{4}}...
The compound formed as a result of oxidation of ethylbenzene by KMnO4 is:
A. acetophenone
B. benzophenone
C. benzoic acid
D. benzaldehyde
Solution
Any aliphatic carbon with hydrogen attached to it, in combination with a benzene ring will be oxidized to benzoic acid regardless of the length of the entire side chain as KMnO4 is a strong oxidizing agent.
Complete step by step answer:
Here, we are provided with ethyl benzene i.e. C6H5CH2CH3that has to be oxidized with KMnO4. KMnO4 is a strong oxidizing reagent. What it does is it will oxidize the carbon that is attached to the benzene ring because the hydrogen attached to that carbon will be the most acidic hydrogen. The two hydrogens attached to the carbon get replaced by two - OH groups. Similarly, in the place of CH3 an - OH group will get attached. As a result, three - OH groups will get attached to the benzene ring. The attachment of three - OH groups will make the product highly unstable due to which it will tend to lose water H2O and the reaction will end with the formation of benzoic acid C6H5COOH as an end product.
The reaction is as follows:
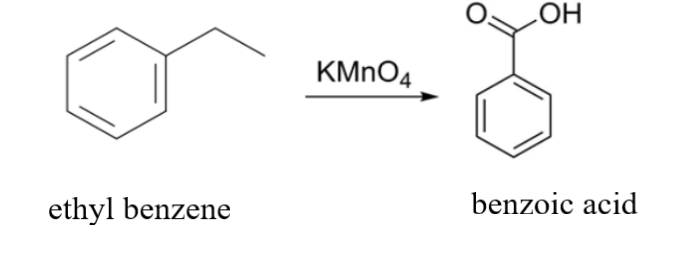
Another example can be:
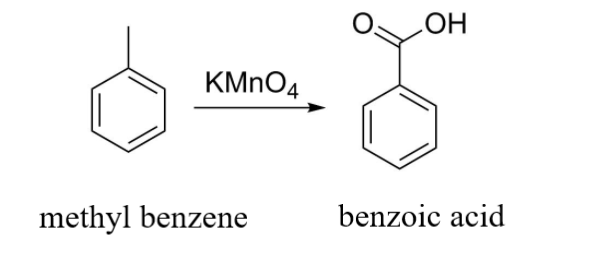
In this example, the result of oxidation of methyl benzene with KMnO4 is benzoic acid.
From the above two cases, we infer that the reaction does not depend on the length of the side chain attached to the benzene ring. Irrespective of the side chain, the KMnO4 will always oxidize it to benzoic acid.
Hence option (C) is correct.
Note:
Keep in mind the trick that no matter how long the side chain is, the result of this reaction will always be benzoic acid as explained above that the reaction does not depend on the length of the side chain attached to the ring.
