Question
Question: The compound formed as a result of oxidation of ethylbenzene by \[{\text{KMn}}{{\text{O}}_{\text{4}}...
The compound formed as a result of oxidation of ethylbenzene by KMnO4 is:
A.benzophenone
B.acetophenone
C.benzoic acid
D.benzyl alcohol
Solution
Ethyl benzene is a cyclic compound. On undergoing oxidation in the presence of KMnO4, the product will be also a cyclic compound.
Complete step by step solution:
We know that the formula of ethyl benzene is C8H10. We will see what this compound will give in the presence of KMnO4.
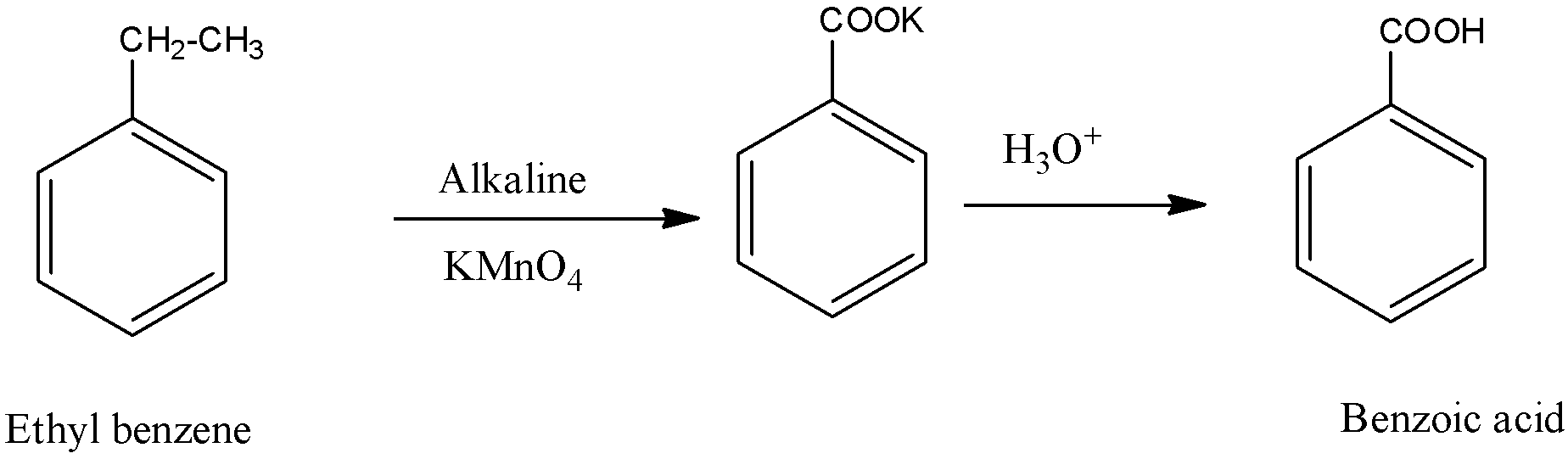
In the above reaction, we see that ethyl alcohol gives benzoic acid during the oxidation of ethyl benzene by KMnO4.
Therefore,C is the correct option.
Additional information:
KMnO4 is a very common reagent used in the oxidation of various compounds. We can draw the structure of potassium permanganate as follows.
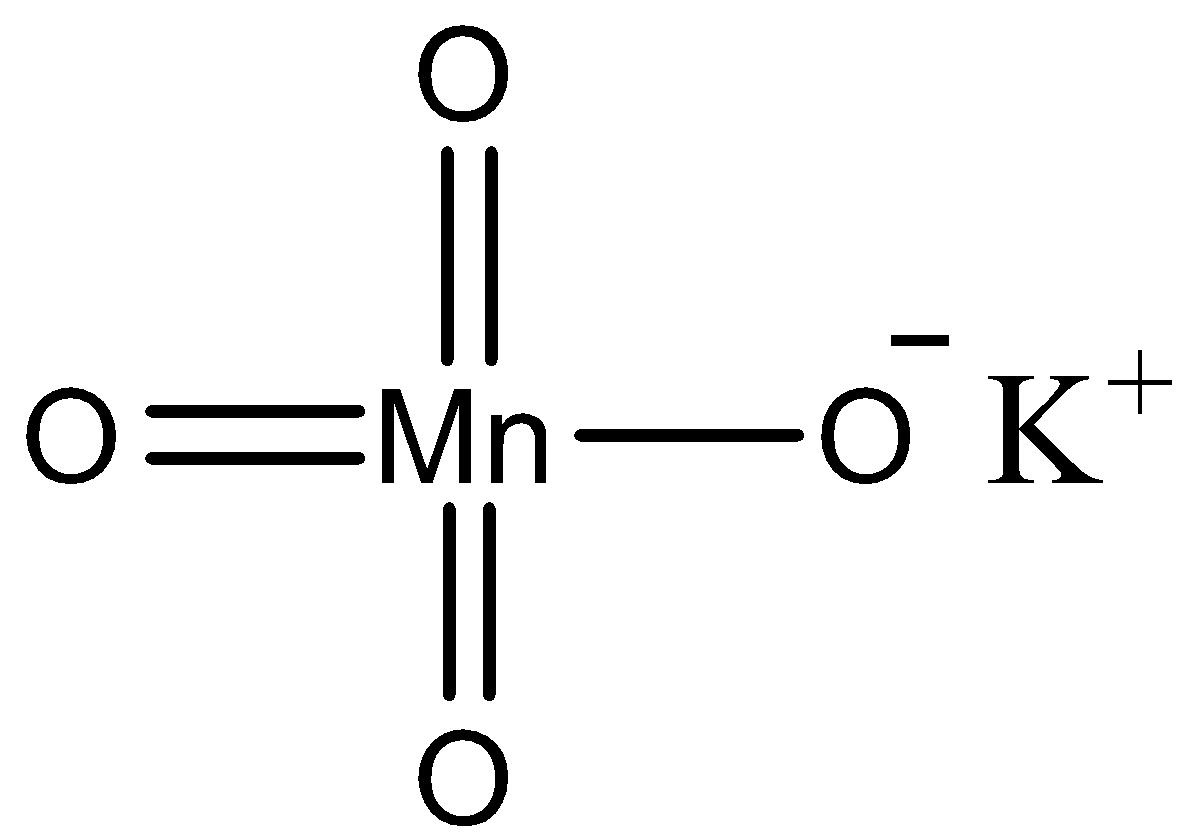
KMnO4 is an odourless, purple coloured crystalline solid. It is soluble in water, acetic acid, acetone, methanol, pyridine etc. It is soluble in solvents like ethanol and in other organic solvents. Potassium permanganate exists in the form of monoclinic prisms. KMnO4 is opaque with a blue metallic lustre. Potassium permanganate is a strong oxidizing agent, and it is used as an oxidant in a lot of chemical reactions.
When potassium permanganate in solid form is heated, it undergoes decomposition. The reaction is as follows:
2KMnO4→ K2MnO4+ MnO2(s) + O2
On heating with an alkali, potassium permanganate it produces manganese and oxygen gas is evolved.
4KMnO4+ 4KOH→ 4K2MnO4+ 2H2O + O2
Note: In qualitative analysis, Potassium permanganate is used for determining the permanganate value. It is also used in the treatment of various bacterial infections.
