Question
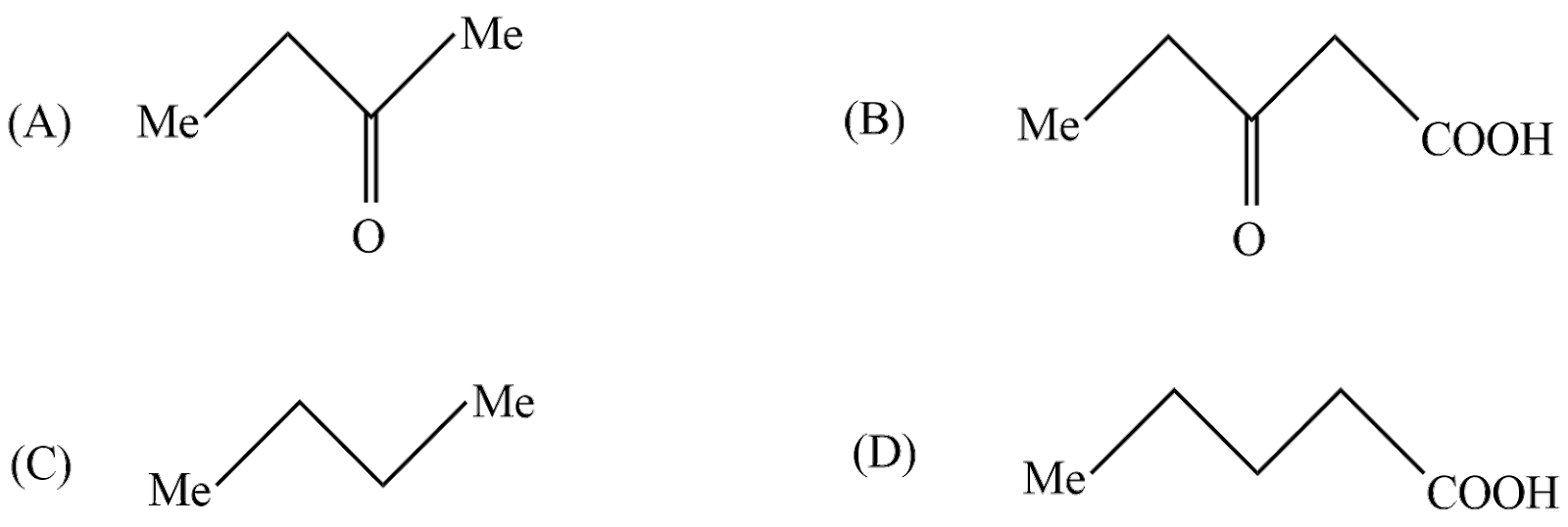
Question: The compound (C) is:  is:
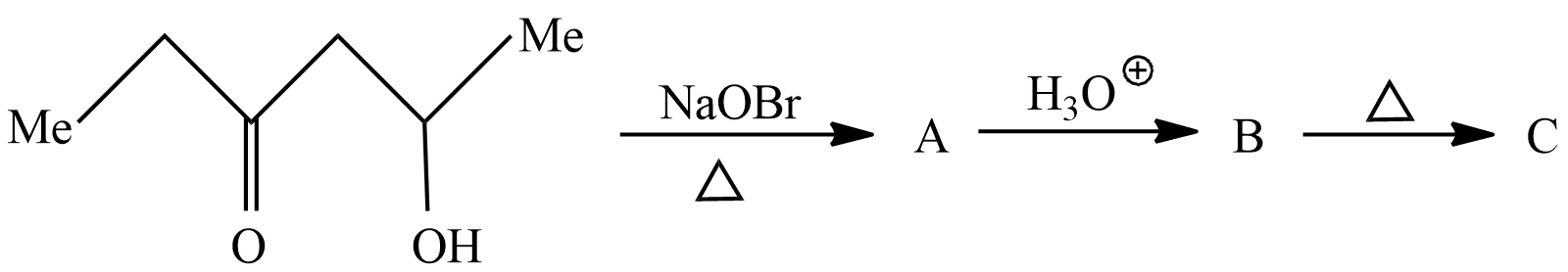
Solution
Hint : The compound given here is 5−Hydroxy−3−hexanone which is treated with Sodium hypobromite to give product A which then is treated with hydronium ion to give product B. Product B is heated to obtain product C. we will see each reaction to understand the process and identify the products formed from each of the reaction.
Complete Step By Step Answer:
In the first reaction 5−Hydroxy−3−hexanoneistreatedwithSodiumhypobromiteitwillremoveamethylgroupandformalkylhalideandSodiumHydroxide.ThereactiontakingplacehereiswrittenasIntheabovereaction 5 - Hydroxy - 3 - hexanone reactswithSodiumhypobromitetogiveMethylbromideand 5 - Hydroxy - 3 - pentanone .So,theproductAaccordingtoreactionis 5 - Hydroxy - 3 - pentanone .Let′sseethesecondreactioninwhichproductAreactswithhydroniumion;inanoxidationreactionthealcoholisconvertedtocarboxylicacid.ThereactiontakingplacehereiswrittenasInabovereaction5−hydroxy−2−pentanoneundergoesoxidationreactiontoform 3 - Oxopentanoicacid .So,theproductBaccordingtoquestionis 5 - Hydroxy - 3 - pentanone Let′sseethelastreactioninwhichproductBundergoesstrongheatingtoremovecarbondioxide.Itisalsoknownasthedecarboxylationreaction.thereactiontakingplacehereiswrittenasInabovereaction 3 - OxopentanoicacidundergoesdecarboxylationreactioninstrongheatingconditionstoformCarbondioxideand {\text{n}} - butanone .So,accordingtothequestiontheproductCis {\text{n}} - butanone $ .
Note :
Carefully observe the position of the functional group while writing a reaction because it is necessary for the product formation. Also pay attention to the conditions in which the reaction is carried out because it can affect the reaction.
