Question
Question: The compound \({{C}_{7}}{{H}_{8}}\) undergoes the following reactions: \({{C}_{7}}{{H}_{8}}\text{ ...
The compound C7H8 undergoes the following reactions:
C7H8 3Cl2/Δ A Br2/Fe B Zn/HCl C. The product C is:
(A) 3 - bromo -2,4,6 - trichlorotoluene
(B) m - bromotoluene
(C) p - bromotoluene
(D) o - bromotoluene
Solution
Draw the expanded structure of the compound C7H8. Chlorine acts as a substituting group that will replace hydrogen atoms attached to the carbon atom. Bromine in the presence of iron generates an electrophile. Now zinc in the presence of HCl substitutes the chlorine atoms with hydrogen.
Complete step by step answer:
We will first draw the structure of C7H8. The degree of unsaturation present in the organic compounds is 4.
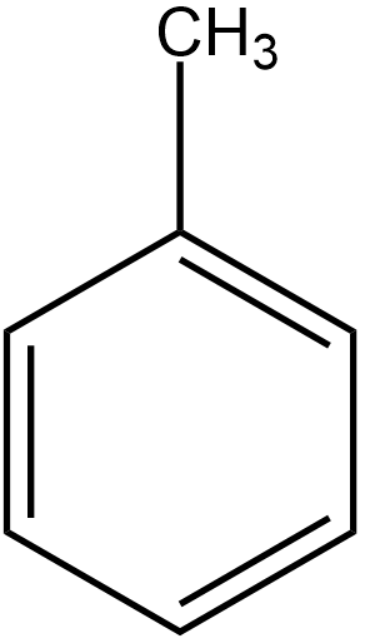
Chlorine in the presence of heat will replace the hydrogen atoms present in the methyl group to form methyl trichloride group. This becomes compound (A).
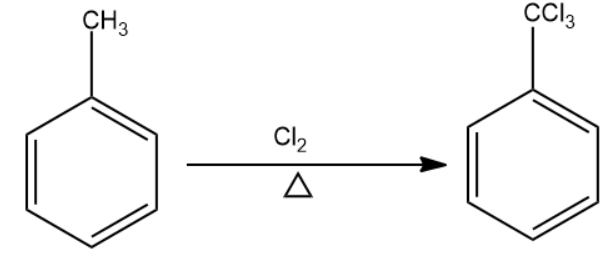
The compound formed reacts with the next reagent which is Br2/Fe. The reagent forms a bromine electrophile which attaches at the meta position of the aromatic compound. This is compound
(B).
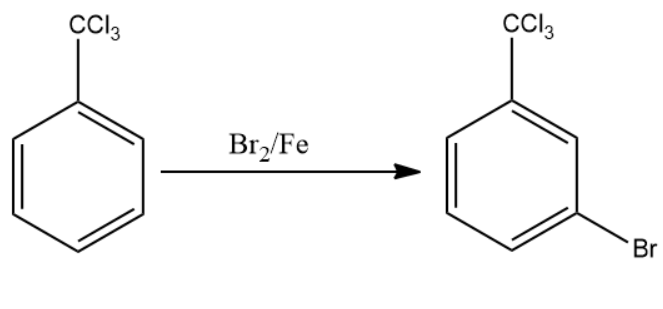
Once product (B) is formed, the compound is made to react with anhydrous zinc chloride in the presence of hydrochloric acid. This reagent replaces the chlorine atoms present with the external carbon atoms with the hydrogen atom from hydrochloric acid. This is compound (C).
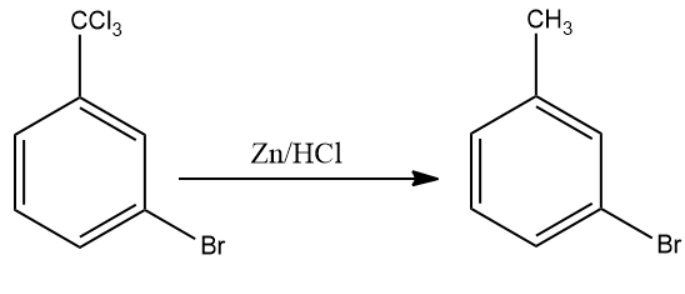
The final product formed i.e. compound (C) is m - bromotoluene.
So, the correct answer is “Option B”.
Note: It is important to know that the common name of an aromatic compound is also considered as the IUPAC name of the compound. For example, toluene is considered the IUPAC name along with methyl benzene for the same aromatic compound.
