Question
Question: The compound \(buta - 1,2 - dien\) consists of: A.only \(sp - \) hybridized carbon atom B.only \...
The compound buta−1,2−dien consists of:
A.only sp− hybridized carbon atom
B.only sp2 hybridized carbon atom
C.both sp and sp2 hybridized carbon atom
D.sp , sp2 and sp3 hybridized carbon atom
Solution
Hybridization can be defined as the mixing of two atomic orbitals with the same energy levels to give a new degenerated type of orbitals. There are different types of hybridization namely: sp,sp2,sp3,sp3dand sp3d2 .
Complete step by step answer:
-Hybridization can be defined as the mixing of two atomic orbitals with the same energy levels to give a new degenerated type of orbitals. There are different types of hybridization namely: sp,sp2,sp3,sp3d and sp3d2 .
-We will discuss about each hybridization as follows:
-sp hybridization: In this type of hybridization, one s orbital and one p orbital are mixed together to form a completely new orbital called sp orbital.
It forms linear molecules.
Example of sp hybridization: BeF2,BeCl2 .
-sp2 hybridization: In this type of hybridization, one s orbital and two p orbital are mixed together to form a completely new orbital called sp2 orbital.
It forms a trigonal symmetry.
Example of sp2 hybridization:BF3,BH3 .
-sp3 hybridization: In this type of hybridization, one s orbital and three p orbital are mixed together to form a completely new orbital called sp3 orbital.
It forms a tetrahedron.
Example of sp2 hybridization: ethane,methane.
-sp3d hybridization: In this type of hybridization, one s orbital , three p orbital and one d orbital are mixed together to form a completely new orbital called sp3dorbital.
It forms a trigonal bipyramidal.
Example of sp2hybridization: PCl5
-sp3d hybridization: In this type of hybridization, one s orbital , three p orbital and two d orbital are mixed together to form a completely new orbital called sp3d2orbital.
It forms an octahedron.
Now, we will discuss about the compound buta−1,2−dien :
It is an organic compound and it is the isomer of 1,3−butadiene .
The structure of buta−1,2−dien is given below:
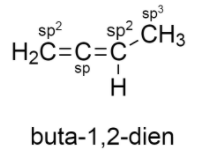
It consists of two double bonds and two sigma bonds.
-CH2 is attached to three atoms: two of hydrogen, one to carbon atom. Thus it is sp2 hybridized.
-The carbon adjacent to the CH2 group also has two double bonds which means it is sp hybridized.
-The carbon that is attached to CH3 is sp2 hybridized because it is bonded to two carbon atoms and one hydrogen atom.
The carbon atom of CH3 is sp3 hybridized.
So the correct is option D) sp , sp2 and sp3 hybridized carbon atoms.
Note:
The basic criteria to check for hybridisation of carbon atoms is to look at the number of bonds it has like if a carbon atom has triple bond it is sp hybridised , if it has double bond it is sp2 hybridised and if a carbon atom has only single bonds it is sp3 hybridised .
